Species are recorded to the highest taxonomic level possible, following
Species can be added easily, and there is a wide range of British mammals, birds, fish, amphibians and reptiles in the system by default (as standard taxon abbreviations, Latin names and common names – together with order and family names). Broader taxonomic levels (e.g. family and genus) are provided, as are groupings such as 'large mammal' and 'small mammal', as these are still of analytical significance (e.g. Uerpmann 1973, 309). Most groupings are self-explanatory, but 'large mammal' refers to animals of horse, cow or large cervid size, 'medium mammal 1' refers to animals of sheep, pig or small cervid size, 'medium mammal 2' refers to animals of cat, dog or hare size and 'small mammal' refers to animals of rat or mouse size.
Inter-analyst variability in taxonomic identification is a critical, if inevitable, aspect of zooarchaeology (Driver 1991; Gobalet 2001). This problem is likely to remain one of the most significant weaknesses in data shared via the York System and similar databases. It is clearly of equal significance to zooarchaeological data disseminated in more traditional formats. Nevertheless, the danger of na�ve use of easy to download information must be recognised.
Zooarchaeologists often choose not to identify every single bone from an assemblage, instead selecting a series of diagnostic elements that occur frequently, are of zooarchaeological value, and can be readily identified (Davis 1987, 35; Colley 1990, 212; Reitz and Wing 1999, 155). Such an approach is often able to produce a high quantity of data with minimal effort (Davis 1992, 1). Although not always overt, these bones may be given a name or 'quantification code' (Barrett and Oltmann 1997, 620). Zooarchaeologists may also have a further list of elements that are recorded in special cases, if resources permit, or if those bones contain valuable zooarchaeological data relating to butchery marks, pathologies, or non-metrical variations. Again, this second set of bones may be given a different name or quantification code. The quantification code (QC) of an element is a measure of its zooarchaeological value.
In the original EAU recording protocol, specimens from mammals and birds that had designated diagnostic zones were classified as 'A' bones, while any remaining elements that could be identified at the species level were termed 'B' bones (after S. Payne, pers. comm.). All other specimens that could not be identified to species were grouped into various categories, such as large or small mammal (Dobney et al. 1999).
Barrett's fish recording protocol originally contained a series of five categories (0 to 4), but when this was formalised into Fish 1.1, the number of categories was reduced to four (0, 1, 2 and 4):
For the purposes of the York System, quantification codes have been formalised into these four categories. Bones that had been termed 'A' class are now QC 1, while those that were 'B' class are now mostly QC 0. Certain unusual, interesting, or rarely occurring bones have been classified QC 4 (including antler, fish otoliths, etc.). Quantification code 3 has not been used, while QC 2 is only used for fish vertebrae, the mineralised vertebral centra of cartilaginous fishes and sturgeon scutes. The full listing is provided in the help files in the database.
It is worth noting that fish element nomenclature has varied substantially over the last century. Wheeler and Jones' (1989) table 7.1 lists some of the different naming systems in current use. The York System follows their 'recommended' nomenclature.
As specimens are very rarely entire bones, some system must be in place to record which part of a bone is present. This can either be a description (e.g. 'proximal articular surface plus part of shaft'), or a string of characters linked to a predetermined coding system. The latter is not intuitively easy to use, but this problem has been addressed in the York System by employing a graphical ('point-and-click') interface.
The concept of recording the diagnostic zones present for a specimen originated with Watson's work on assemblages from Cyprus (1979). A variety of zone definitions have since been published, in whole or in part (e.g. Münzel 1986; Rackham 1986; Dobney and Rielly 1988; Serjeantson 1991; Morlan 1994; Cohen and Serjeantson 1996; Moreno-Garcia et al. 1996; Newton et al. 2001) and many more unpublished systems are no doubt in use.
Watson introduced the diagnostic zone as an attempt to limit the number of bones that were counted twice during the quantification stage. He recommended that a diagnostic zone 'should be as species-specific and as commonly preserved as possible, suitable for unfused as well as fused material, as free as possible from age-biases and as rarely as possible broken or split. The vital rule is that it must not be possible to count the same bone twice with any one zone' (1979, 129). Therefore more than 50% of a zone must be present before it is recorded in the York System. Watson comments that '[s]tandardization of the list of zones will not be achieved easily, but it should be possible for ... comparisons to be made... When more work has been done to establish the most useful zones, it may be advantageous to give each zone a permanent reference number' (1979, 132).
Although the advantages of such a system are obvious, particularly for computer based recording and analysis, the choice of zone definitions is not simple. Some zones may combine parts of an element which are likely to fragment into more than one identifiable specimen. Others are unlikely to be identified to a meaningful taxonomic level unless still attached to a more distinctive neighbouring zone. Yet others are coded in a seemingly random order over the surface of each element, making them difficult to remember and use in practice. There is also a clear problem of taxonomic variability in bone morphology. At what level does one provide diagnostic zone diagrams: class, order or family?
The mammal and bird diagnostic zones used at York directly follow the EAU protocol, with some additions and minor changes (numerical codes of greater than nine have been changed to letters to limit confusion, for example), while fish diagnostic zones follow Fish 1.1 (after Barrett 1995; Barrett and Oltmann 1997). An example showing the zones of a horse femur is illustrated below. A complete set of the illustrations used can be viewed in, or printed from, the help file.
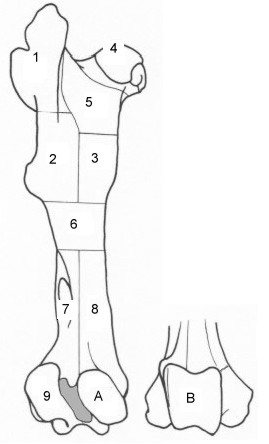
Figure 5: Diagnostic zone example (J.F. Harland)
The EAU and Fish 1.1 diagnostic zone diagrams we have used in the York System exhibit many of the problems discussed above. In particular, the mammal bone diagrams are not coded in an easy to follow format and only one family of fish (Gadidae) is illustrated. It was decided to retain these systems despite their problems partly to ensure backward comparability with previous 'in-house' work, partly because there was not a clearly superior alternative and partly because their worst deficiencies can be mitigated. The complexity of the mammal bone coding, for example, is made irrelevant by inclusion of a graphical interface in the York System (although 'old hands' may find it quicker to directly type in the zones present). The problem of variability in fish anatomy is less easily resolved without including a clumsy number of alternate zone diagrams in the programme. As a compromise solution, a series of illustrations for other common orders and families of fishes is currently in preparation. When published as 'hard copy', it is recommended that they be used alongside the York System to indicate how the broad model provided by the gadid diagrams should be modified for taxa with different bone morphologies.
Element fragmentation is often measured using the number of diagnostic zones represented by each specimen (e.g. Serjeantson 1991) or a broad semi-quantitative estimate of the percentage of a complete element remaining (e.g. Marean 1991; Nicholson 1992; Barrett and Oltmann 1997, 629–30; Zohar et al. 2001, 1048). Both are possible with the York System, but given that not every species and element combination will have a diagnostic zone, a separate record is recommended in the 'completeness' field. This allows estimates with values ranging from '0 to 20% complete' to '81 to 100% complete' (following Fish 1.1). Perhaps surprisingly, an inter-analyst trial of ten zooarchaeologists has shown completeness estimates of this kind to exhibit less variability than a fragmentation index based on diagnostic zones (Barrett 1998).
The anatomical side of each element is recorded as left, right and midline or unknown.
In mammals and birds, age is typically assessed through tooth wear stages and epiphyseal fusion data. For teeth, the main domestic species are typically recorded using either Grant's (1982) or Payne's (1973; 1987) methods, both of which are incorporated in the York System. The former is used to record pig and cattle dentition, while the latter is used for caprines.
In the case of epiphyseal fusion, the fusion ages of modern animals are applied to specimens recovered archaeologically (e.g. Silver 1969; Moran and O'Connor 1994; Davis 2000). The York System records the fusion state for each epiphysis using 'unfused', 'fusing' or 'fused'. A separate record may also be made in the absence of epiphyses based on general morphology and size, including the categories 'neonatal', 'juvenile', 'sub-adult' and 'adult'. These cannot be precisely defined given the diversity of variables involved between elements and taxa. Comparison with reference material of known age is often the best guide, assuming that differences in size between ancient and modern animals are kept in mind.
General age categories for horn cores may be recorded in the York System, following Armitage (1982).
Fish continue to grow throughout their lives, so the size of a fish can provide an approximate indication of its relative age (e.g. Mellars and Wilkinson 1980). However, this is greatly dependent on the comparative data available and the population being studied (Wheeler and Jones 1989, 147). Following Fish 1.1, fish total length is recorded using six general categories (Table 1), based on the sizes of known reference specimens.
| Category | Metrical equivalent |
|---|---|
| Tiny | 1–150mm |
| Small | 151–300mm |
| Medium | 301–500mm |
| Large | 501–800mm |
| X Large | 801–1000mm |
| XX Large | >1000mm |
Table 1: Fish sizes
Regression equations applied to fish element measurements can also be used to estimate fish total length or weight during the analytical process (e.g. Enghoff 1983; Desse and Desse-Berset 1996).
Morphological characteristics reflecting the sex of an individual can be difficult to detect during primary identification. Certain diagnostic bones reflect sex, including the presence of distinctive canines in male pigs and red deer, the presence of a baculum in males of some species, the presence of medullary bone in the long bones of female birds during egg laying and the presence of tarsometatarsal spurs in some male birds (Reitz and Wing 1999, 168; d'Errico and Vanhaeren 2002). Other features linked to sex, but with less certainty than the above, include the presence of canines in stallions, the absence of horn cores in female ungulates, and the absence of antlers in female deer (with the exception of the reindeer) (Davis 1987, 44). Any such features can be noted during the recording process in the general notes field and some will be recorded as a matter of course as element (e.g. a baculum) or tooth wear (a pig or horse canine) data. With these exceptions, there is no specific field in which to record sex given the rarity of secure attributes. Statistical methods can be employed to determine sex using metrical data (see below) during later analyses, although the presence of castrates complicates matters (e.g. Davis 2000).
Unusually for zooarchaeologists, there is widespread acceptance of von den Driesch's (1976) system of measurements for mammals and birds. Of course, variations and additions exist (see Reitz and Wing 1999, 169), and any extra measurements that have been deemed necessary are described or illustrated with a diagram in the York system's help files.
Fish measurements are much less standardised. Because mammal and bird metric data are often used to assess domestication, sex and 'breed' – not applicable to fish – only a few measurements ever need be recorded (Wheeler and Jones 1989, 139–41). For example, otolith measurements can reflect seasonality on a site (Mellars and Wilkinson 1980), while regression equations can be applied to a few bones to reconstruct fish size (Desse and Desse-Berset 1996). Following Fish 1.1, a selection of measurements (illustrated in the help files) are recorded for ten elements, based on Enghoff (1994), Morales and Rosenlund (1979), Watt et al. (1997) and Jones (1991). As with all aspects of the database, additional measurements can be added. A qualitative estimation of fish size (total length) can also be recorded as noted above.
The maximum linear dimension (MLD) of a specimen can also be recorded. This is a measure of its longest axis. It can be used to assess the general fragmentation of an assemblage (in conjunction with diagnostic zone data, percent completeness estimates and context-level fragmentation data) (e.g. Lyman 1994b, 334–35; Outram 2001). MLD is, however, a relatively labour-intensive measure and its role as a taphonomic indicator can be biased by inter-assemblage differences in recovery methods and animal size. Some users may thus choose to leave this field blank when specimen size is not directly related to their research questions.
Many zooarchaeologists do not record the weight of individual specimens because it is assumed that a variety of factors are likely to bias data of this kind (see Casteel 1978; Reitz and Wing 1999, 170, and references therein). Nevertheless, bone weight can be a valuable tool, particularly for inter-class comparison (Barrett 1993) and as another index of fragmentation (Outram 2001). The York System gives users the opportunity to record weight at either the specimen or the context level.
Pathological studies in zooarchaeology are less advanced than in human osteoarchaeology, particularly in the case of fish because 'little is known about which diseases of fish can be detected from a study of bones... there is no body of data... which links diseases to features present on fish bones' (Wheeler and Jones 1989, 120). In the York System, a description of each pathology may be entered and its location recorded using the diagnostic zone system. More experienced zooarchaeologists may choose to assign a diagnosis at this stage. Dental enamel hypoplasia, for example, can be systematically recorded (see Dobney and Ervynck 2000) and a number of other common pathological conditions are 'pre-entered' in the system. However, O'Connor recommends that 'the emphasis is put on describing and not simply diagnosing the lesions concerned' (O'Connor 2000, 108; his italics). Drawings or photographs may be helpful in later analysis. There is no provision for recording them as part of the York System, but a reference could be made to a file stored elsewhere.
Non-metrical variations can encompass a range of observations, including, for example, the congenital absence of certain teeth (O'Connor 2000, 119–21) or the location of the major nutrient foramen of a sheep femur (Noddle 1978). These may be recorded using a description and location (following the diagnostic zone system if desired), and again, photos or drawings may prove useful.
Butchery marks can be relatively easy to identify and record (Blumenschine et al. 1996; Greenfield 1999). They have long been used to address questions relating to hunting practices, husbandry regimes, fishing strategies, trade and exchange (see Lyman 1994b, 294–353; Barrett 1997, 627–28). Tooth marks left by rodents, carnivores or even ungulates can also be distinguished (Lyman 1994b, 194–97, 205–16; Brothwell 1976). The York System records the presence, type and (using the diagnostic zone system) general location of both butchery and gnawing marks. However, drawings are probably the best way to record the precise location of cut marks. They may be incorporated into a future version of the database, but at present file cards with schematic representations of common elements are still used for detailed recording of butchery at York.
Bone 'preservation' is a general category that is typically used to record bone tissue integrity (i.e. weathering or texture), the level of fragmentation present, or some combination of these two variables (e.g. Serjeantson 1991; Dobney et al. 1996, 18–19). Although approaches can vary greatly, many zooarchaeologists create an ordinal scale of up to six categories, sometimes a derivative of Behrensmeyer's weathering stages (1978). The categories now used at York refer exclusively to bone tissue integrity (defined as texture). They are:
It is important to be aware that the rough surface known as 'juvenile cortex' on the bones of very young animals should not be mistaken for poor preservation and thus a low texture score (although a specimen can exhibit both juvenile cortex and poor preservation).
It is well known that recovery methods influence zooarchaeological results (Payne 1972; Clason and Prummel 1977; Jones 1982; Stewart 1991; Vale and Gargett 2002). This variable can be recorded at both the specimen and the context level, in order to ensure that it can be recorded in all circumstances (when, for example, different sieve size fractions exist for material from the same archaeological layer).
Other variables that may be recorded at the specimen level include acid etching following partial digestion (e.g. Wheeler and Jones 1989, 67, 73; Stallibrass 1990), root etching (e.g. Lyman 1994b, 375–77) and burning (e.g. Shipman et al. 1984; Nicholson 1993; 1995).
© Internet Archaeology
URL: http://intarch.ac.uk/journal/issue13/5/specimen.html
Last updated: Thu Mar 13 2003