The commonest source of cobalt oxide is the mineral asbolane (also known as asbolite) (Hodges 1981, 45), an impure oxide of manganese. Less common is the sulphide, linnaeite. Cobalt introduced to a glass by linnaeite could therefore be expected to have low levels of manganese, while glasses coloured using cobalt from asbolane would contain a relatively high proportion of manganese (Hodges 1981, 204). Sayre (1964, 1-25) distinguished between 'western' Roman cobalt-blue glass containing elevated manganese levels from those in Mesopotamia and south-west Iran, which had significant levels of arsenic, with impurity levels of iron, nickel, copper, tin and lead oxides also supporting these regional provenances. Cobalt is also found associated with nickel, bismuth, arsenic and silver in what are somewhat confusingly known as five-element ores (Ag-Co-Ni-Bi-As ± U), although only Co-Ni arsenides in association with native silver are generally prerequisite for the classification (Kissin 1992), often in the form of minerals such as skutterudite ((Co, Ni, Fe) As2-3), cobaltite (CoAsS), safflorite (CoAs2) and nickeline (NiAs). Occurrences of these ores are probably world-wide (Bastin 1939; Kissin 1992; Badham 1976, 559-71; Ramdohr 1969, 1174), with well-established examples found in North America, central Europe and more recently the Anarak district of Iran (Tarkian et al. 1983).

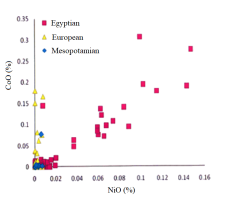
Cobalt has been found in association with manganese, zinc, nickel and alumina in Egyptian New Kingdom vitreous materials (Kaczmarczyk and Hedges 1983) with elevated levels being present in cobalt-coloured glasses. Figure 2 shows the relationship between cobalt and nickel for Egyptian glasses (LBA Egyptian and 'early' 1st millennium BC) compared to Italian and Greek glass from the 10th to the 7th centuries BC and a few samples from Ur, Mesopotamia (10th-6th centuries BC) (Duckworth 2011, 146). It can be seen that CoO increases almost linearly with NiO for Egyptian glasses (apart from one find - dated between the 8th-5th centuries BC), indicating that both have been introduced into the glasses from the same ore source. However, the turquoise, blue and black samples from Italy (10th-7th centuries BC), do not show the same correlation, indicating that the cobalt in these samples was from another source.
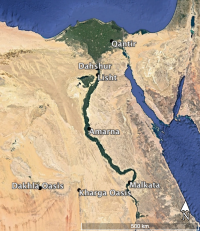
Cobalt-bearing alums found in the oases of the Western Desert of Egypt are thought the most likely source of the cobalt used to colour LBA Egyptian glass (Shortland 2000) (Figure 3), despite these minerals being a dull pink colour and not the deep blue found in glass. Nevertheless, this source was initially proposed by Farnsworth and Ritchie (1938), who realised that cobalt was a deliberate additive to 18th Dynasty cobalt-blue glass, thereby suggesting an Egyptian alum source might have been used as the source of the cobalt-bearing mineral. Garner (1956) also suggested that a cobalt-bearing ore was used for the colouration of Egyptian glass. Lucas (1962) suggested a Persian source for a cobalt colourant, with Stern and Schlick-Nolte (1994) also endorsing a source in Iran or Asia Minor. Dayton (1981; 1993, 38) suggested a central European source, although this was rejected by Kaczmarczyck and Hedges (1983, 301-2) and Nikita and Henderson (2006). Kaczmarczyck (1986) and Rehren (2001) supported the premise that cobalt-bearing alum from the Western Oases was the source of Egyptian cobalt, with the Kharga and Dakhla oases considered to be the most likely origin of the cobalt used to colour LBA Egyptian glass (Shortland 2000).
Glass compositional signatures, however, are not entirely consistent with either oasis. The ED-XRF (energy dispersive x-ray fluorescence) analyses on which Kaczmarczyk (1986, 369-76) based his interpretation were, to some extent, questioned by Lilyquist and Brill (1993) as the relative levels of the impurities in the final glass were not consistent with this view. For example, the elevated levels of alumina, which were assumed to be a consequence of the cobalt-bearing alum source, were found in all 21 cobalt-blue glasses (>1.2%Al2O3) they analysed but were also present in seven of the 43 Egyptian non-cobalt glasses. Furthermore, cobalt-blue glass has low potash levels (i.e. K2O), something shared by both New Kingdom and cobalt-blue glass found at Nimrud (Reade et al. 2005, 23-27). Shortland and Tite (2000) argued that the low K2O levels in the Egyptian glass indicated that the soda source derived from natron, as plant ash imparts a concentration well in excess of 1.5%K2O. The high magnesium content of these glasses was considered to result from the cobalt alum. This view was challenged, however, by Rehren (2001) who advocated a plant ash source. Tite and Shortland (2003) modified their position, suggesting that Egyptian cobalt glass contained both natron and plant ash. Reade et al. (2005, 23-27) considered the early 1st millennium BC Nimrud glass derived its soda from natron as its lime content was much lower than that expected in glass where the alkali is added in the form of plant ash. This leaves an open question regarding the soda source in the Egyptian glass, as these glasses contain high lime. The alum sources of Egypt's Western desert contain effectively no lime.
Several ideas have been proposed to explain the disparities between these alum sources and New Kingdom cobalt glass compositions. These are based on the modification of the alum source material, such as roasting the ores (Henderson 2013, 71), converting the alum into a sulphate (Kaczmarczyk 1986, 369-76; Noll 1981, 143-54) or other methods that concentrate the alum prior to its addition in glass-making (Shortland et al. 2006a). However, although low concentrations of cobalt (Co2+) are needed to colour glass (i.e. 0.05% cobalt oxide (CoO) is sufficient to produce a rich, deep blue (Henderson 2000), compared to 1-2% CuO), these alums have very low concentrations of cobalt, with CoO/Al203 levels being around four times lower in the alums than in cobalt-blue glass and frit (semi-reacted glass) (Tite and Shortland 2003). Furthermore, experiments to concentrate the cobalt have failed to reproduce the ratios found in archaeological glass and frit, which suggests that large amounts of alum would have needed to be added or that alums from these sources were much richer in cobalt in the past (Shortland et al. 2006a). However, in order to accommodate the larger amounts of alum in the crucible required to attain the archaeologically attested amounts of cobalt, the amount of plant ash in the recipe would have to be decreased, thereby explaining the low levels of K2O in the cobalt-blue glass but not the comparable levels of MgO found in non-cobalt glasses (Rehren 2001). Alternatively, adopting Occam's razor, it is reasonable to suggest that the New Kingdom cobalt may not have derived from the alum deposits of the Western Desert. This is discussed in Section 4.
The way that cobalt, or any other colourant, was introduced to colour ancient glass has not yet been fully resolved. Early glass was made by heating a mixture of silica, which acts as a network former, soda (plant ash or natron) as a flux to reduce the melting temperature of the silica, and lime, which acts a stabiliser preventing the glass product from dissolving in water, in a furnace to around 1000°C. Lime was introduced in the form of shell with the silica source, possibly inadvertently. Furnace temperatures were achieved by burning wood, or a waste product of some other process (e.g. pressed olive cakes from olive oil manufacturing), thereby introducing contaminants such as potash and phosphorus oxides into the system (Paynter 2009). Furthermore, the presence of iron compounds, often associated with the silica source, caused glass to take on a blue-green hue, which along with entrapped bubbles, airborne carbon particles from the burning fuel and particulate material deriving from the furnace walls, crucibles, shaping utensils and workshop areas, potentially resulted in the production of a dirty, translucent glass.
These aesthetic problems can be alleviated by colouring and opacifying the glass, and by first making a glass frit. Opaque glass in New Kingdom Egypt was achieved by introducing antimony compounds into the base glass (Shortland 2002). The making of a frit has been considered to be an intermediate step in the production of glass (Petrie 1909, 124), allowing bubbles and any contamination to be removed before reheating the frit to make glass proper (Nicholson 2007, 121). Most of the earliest glass was opaque and coloured and of sufficient quality to be used for luxury items. For example, during the 18th Dynasty in Egypt coloured containers were used for perfume or cosmetics (Shortland 2000; Nicholson and Henderson 2000, 195-205).
It is possible that both colourants and opacifiers were added during the frit-forming process. A mechanism has been proposed whereby calcium antimonate crystals were mixed directly into a translucent glass (Lahlil et al. 2010). Semi-finished white opacified glass has been recovered from Amarna that suggests calcium antimonate had been added to a base glass to make a semi-reacted glass frit. Whether this frit was produced at Amarna or had arrived there through trade is uncertain (Smirnou and Rehren 2011). White opaque glass would require the addition of cobalt (blue), lead (yellow) and copper (blue/green) compounds to attain the basic colour palette used for early glass vessels and beads (red requires different processing conditions). Renormalised compositions of yellow Roman glass from addition of lead antimonate (i.e. after subtraction of lead, antimony and iron oxides) were found to have elevated levels of silica (and lower values of lime, potash and magnesia, relative to the renormalised compositions for other colours). It was proposed that a yellow colourant was added to colourless glass in the form of a lead-antimony-silica mixture (called anima to suggest parallels with the Venetian production of yellow glass in the 18th and 19th centuries AD) (Freestone and Stapleton 2013). Similarly, a calculation of reduced compositions from analytical data for 1st to 4th century AD Roman glass published by Mass et al. (1998) shows that, as compared to white and turquoise glasses, opaque yellow and green glasses, the latter coloured by a combination of lead antimonate and copper oxide, exhibit elevated silica contents. Yellow glass from the New Kingdom period does not exhibit the same reduced compositions (Shortland and Eremin 2006), suggesting that the pigment was added directly into the base glass. However, these results are not completely unambiguous, with three of the 19 samples tested having elevated levels of silica with respect to the main group (possibly indicating that the pigment was introduced in the form of a vitreous material). This could suggest that the other 16 yellow samples were prepared by introducing the pigment directly into the glass. Alternatively, it could suggest that if dilution of glass was conducted during recycling (i.e. broken yellow glass with high silica levels was added to glass with lower silica levels to produce a mixture), the three outliers could represent the original undiluted glass.
Addition of a colourant in the form of a vitreous material into a base glass (rather than adding a mineral or its precursors directly to a base glass) circumvents the difficulties surrounding the addition, mixing and even distribution of low amounts of additive into larger volumes, i.e. the bulk base glass melt or the silica-soda-lime precursors. Streaking found in yellow Egyptian glass has been considered to be related to the relatively high viscosity and the resultant difficulty in mixing in the pigment (Molina Giralt 2014, 47). This would be particularly pertinent for the addition of cobalt where only a few hundred ppm are required to colour the glass deep blue, as long as it is distributed homogeneously. In effect, 1kg of glass (around the mass of a glass ingot) requires only around 0.05kg of CoO (assuming that cobalt was readily available in its oxide form), which would need to be distributed throughout the bulk. Using cobalt glass frit, such as that found at Amarna (see Figure 4a), would not only introduce a granular additive of a similar composition to the base glass solvent but also provide a much greater volume of material to distribute, thereby providing a larger area of interaction to colour the base glass evenly.



Traditionally assumed to represent the first stage of glass manufacture (Petrie 1894, 25-6) (Petrie speaks of frit as a colourant and then goes on to place it in the context of glass manufacture), cobalt-blue frit found at Amarna O45.1 has an unusual composition: high levels of silica (often with unreacted quartz pushing the silica concentration up to as high as 84% - i.e. greater than faience and glass found at the site), lower levels of soda (average 8.7%) and lime (average 0.7%) than found in glass, and levels of cobalt (average 0.21%) much higher than required to colour glass deep blue (Tite et al. 1998, 111-20; Tite and Shortland 2003). Furthermore, cobalt-blue frit was found in association with 'fritting pans', which have been interpreted as having multifunctional purposes as crucibles for fritting or melting glass and as moulds for glass ingots (Nicholson et al. 1997). These cylindrical vessels were also internally lined with a white slip containing high levels of CaO (probably applied as lime silicates or lime - Rehren and Pusch 1997), perhaps to prevent glass from being contaminated by iron from the clay vessel, and/or to facilitate the release of glass after cooling. This not only suggests that crucibles/melting pots were being reused rather than being deliberately broken to extract the glass, but also that the colourant, in the form of frit, may have been added during this melting process in these crucibles, thereby allowing the low-soda content vitreous colourant to be distributed evenly in the viscous base glass before melting. A fragment of cobalt-coloured semi-finished glass from Nicholson's excavations in Amarna (Jackson and Nicholson 2007, 109, 115) supports the idea that cobalt could have been introduced to a base glass in the form of a frit. Tite and Shortland (2003, 285, 305-6) now suggest that the frit was indeed used in glass colouring, stating 'the compositions of the cobalt-blue glass and frit are consistent with the hypothesis that the cobalt-blue glass was produced from a mixture of cobalt-blue frit with additional plant ash and quartz'. Figure 4 and Figure 5 show examples of blue frit in fritting pans, glass rods and cobalt-blue glass objects recovered at Amarna (18th Dynasty).



In sum, higher levels of silica in some glasses could suggest that colourant was added to a base glass or its precursors at the fritting stage of glass manufacture in the form of a vitreous material. This stage not only allowed bubbles and any contamination to be removed and colourants to be introduced but also permitted the colourant to distribute throughout the base glass during reheating to make glass proper. The frit found in the fritting pans at Amarna O45.1, with its low levels of calcium stabiliser, was probably not used directly for making objects. In fact, its high cobalt concentration suggests that it could be a concentrated vitreous colourant, which only later would be mixed with a base glass. This would suggest that the frit at Amarna was placed in fritting pans for another reason. This is now examined with regard to cobalt-blue glass and frit.
Internet Archaeology is an open access journal based in the Department of Archaeology, University of York. Except where otherwise noted, content from this work may be used under the terms of the Creative Commons Attribution 3.0 (CC BY) Unported licence, which permits unrestricted use, distribution, and reproduction in any medium, provided that attribution to the author(s), the title of the work, the Internet Archaeology journal and the relevant URL/DOI are given.
Terms and Conditions | Legal Statements | Privacy Policy | Cookies Policy | Citing Internet Archaeology
Internet Archaeology content is preserved for the long term with the Archaeology Data Service. Help sustain and support open access publication by donating to our Open Access Archaeology Fund.