Cite this as: Lahtinen, M. 2022 The High-Status Late Medieval Skull Shaped Relic in Turku Cathedral, Finland - a study of its origin with oxygen and strontium isotope analyses, Internet Archaeology 59. https://doi.org/10.11141/ia.59.8

Finland was one of the last areas in Europe that Christianity reached. The earliest churches in Finland were established and built during the late Iron Age (AD 1100-1200) and medieval period (c. AD 1200 onward), but there was contact with Christian communities outside of Finland prior to this. The spread of Christianity to Finland during the medieval period (AD 1200-1500) was closely tied to the establishment of Swedish rule, as well as growth in trade, and the development of new cities and marketplaces. In south-west Finland, the only medieval diocese in the country, construction of Turku Cathedral began in the late 13th century AD (Hiekkanen 2002). It served as a catholic cathedral for several centuries prior to the Swedish reformation in the early 1500s.
Relics, the physical remains of a holy person or associated materials, are objects of importance in many religions, not least in the Roman Catholic Church. At a relatively early stage in the spread of Catholicism throughout Europe, it became commonplace that churches would have at least one altar containing a relic. Naturally, the cathedral of Turku was no exception. With the reformation in the 16th century, the religious significance of relics vanished, and many were lost during the reorganisation of the church according to the doctrines of Lutheran Protestantism. However, in Turku Cathedral south-west Finland, a unique assemblage of medieval relics survived in situ.
The most prominent of the relics in the Turku Cathedral assemblage is the so-called skull relic, assembled from various bones and covered with several layers of cloth (Figure 1). It was found 1929 in a wooden cist at Turku Cathedral, together with other relics and associated materials (Rinne 1932). Although skull relics are not rare within European collections, few have been studied as extensively as the one in Turku Cathedral (Arponen 2015; Arponen et al. 2018a; 2018b; Taavitsainen 2011). However, with the exception of its Chinese silk damask wrapping, the origins of the other fabric and bone components of the relic have not been ascertained (Branting and Lindblom 1928; Lahti 2003). The mystery of the origin of the materials used for the relic is further compounded by the lack of any accompanying authentica (cedula), a parchment or paper tag revealing the name of the saint relating to the relic.
In this article, the results from the first strontium and oxygen isotope composition analysis conducted on the Turku Cathedral relic assemblage, including the bone fragments and linen textiles are presented. Strontium and oxygen isotope analyses are often used in archaeology to aid the study of migration (e.g. Taavitsainen et al. 2018; Lahtinen et al. 2021). Consequently, the same methods should be able to indicate the origin of the constituent osseous and linen materials used in the Turku relic (see for example Bentley 2006). The results would not only reveal the way in which this, and potentially other similar relics were manufactured in the Middle Ages, but also throw light on the ecclesiastic and trade connections of early 14th century Finland and early Christian history in northern Europe.
Although the Turku relic lacks an authentica (cedula), there are two long-standing and popular hypotheses regarding the identity of the saint to whom it supposedly belonged. Both theories are strongly rooted in the nationalist agenda that was popular at the time in which they were put forward. The saint represented by the Turku relic is portrayed in a narrative embroidery sewn on the topmost silk wrapping of the artefact. It has been argued that this embroidery depicts the saint to whom it belonged, in the act of their martyrdom. In 1932, Finnish State Archaeologist Juhani Rinne (1948) interpreted the character depicted in this embroidery as a bishop. As the relic was in Turku Cathedral, he identified the martyr as the patron saint of the cathedral, St Henry of Uppsala, alleged to have been martyred around AD 1156. Rinne thought the mandible of the relic matched a cranium found in 1924 in the walled-up niche of the cathedral sacristy, which he believed to be that of St Henry. The argument is problematic, as in the embroidery the individual depicted is being decapitated with an axe, whereas St Henry was believed to have been executed with a sword (Lahti 2019). This was supported by two Swedish textile researchers (St Henry is believed to be a historical character and was the only Finnish saint and patron saint of Turku Cathedral). However, recent research has called the very existence of St Henry as a real historical figure into question (Heikkilä 2005; 2016). According to folk-legend, still taught at Finnish schools, St Henry was decapitated during his crusade into Finland. The legend of St Henry is still widely known in Finland, and the importance of these relics to the general public has increased as a result of the research and interest shown by the Finnish Catholic church in the Turku Cathedral relic collection. The Finnish Catholic church still has an altar for the saint and Turku Cathedral holds an annual remembrance for the saint.
The second theory regarding the identity of the saint represented by the relic was published in 1954 by another Finnish State Archaeologist, Carl Axel Nordman (1954). The skeleton of King Eric IX of Sweden, or St Eric (the patron saint of Uppsala and Sweden, martyred in AD 1160), kept in a reliquary in Uppsala Cathedral, Sweden, is missing its mandible. Nordman therefore decided to compare the physical properties of the Turku mandible and St Eric's cranium in Uppsala. Although there are distinct differences between the cranium and the mandible, Nordman considered it possible that they originated from one person, St Eric. Furthermore, he saw similarities between the embroidery of the relic and the 13th-century Martyr Cope in Uppsala Cathedral, which Nordman considered as proof that the skull shaped relic originated in Uppsala. However, this would not explain the origin of other skull bones of the relic. Lahti (2019) comments that the scene is more typical of St Eric than Henry as Eric was killed using a sword.
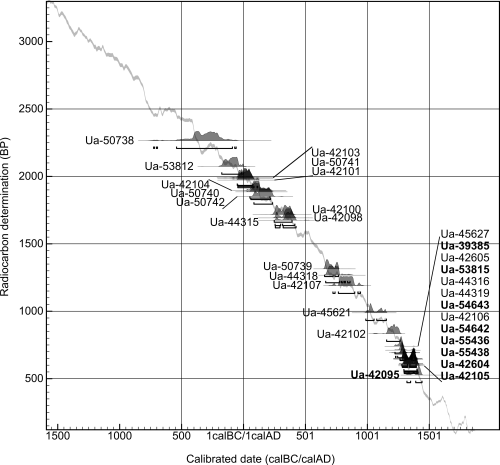
Since 2011, the relic has been studied as part of the Turku Cathedral Relics Research Project of the Turku University Department of Archaeology. The complexity of the relic was initially hinted at by the age of the bones as the results of 14C dating. These cumulatively spanned a timeline of thousands of years, with the oldest dating to the pre-Christian era (Figure 2), and the latest to the end of the 14th century AD (Arponen et al. 2018b). Finally, in 2012 the mandible was 14C dated and the result, 250–300 and 320–430 cal AD (probability 95.4%, 1675±30 BP, Ua-44315), refuting the possibility that it could have belonged to either St Henry or St Eric (Arponen et al. 2018b).
Instead of considering the relic as part of a historic story of national significance, the Turku relic embroidery could just as readily refer to part of a much larger European Christian tradition. The most popular cult associated with skull relics in the Middle Ages was the one of St Ursula and 11,000 virgins (Arponen et al. 2018b) (see more in Nordic countries from Lahti 2019). The cult spread quickly from its centre, Cologne, Germany, throughout much of Europe, especially from the 12th century AD onward. It was also known among the medieval diocese of Turku (Rinne 1948). Its significance, however, was emphasised only in the mid-15th century AD in Turku, when the feast day of 11,000 virgins (or St Ursula) was raised in the local Calendars of Saints from memoria to duplex or totum duplex, the two highest ranks of feast days (Malin 1925, 168–69). The importance of the cult was highlighted in Turku Cathedral when an altar with a chapel dedicated to the 11,000 virgins was founded in 1455 (Rinne 1948; Arponen et al . 2018b).
In general, the skull relics related to the cult of 11,000 virgins resemble the relic in Turku Cathedral, but there are some notable differences, both in structure and appearance. In most cases, the skull relics of 11,000 virgins comprise wholly or partially intact crania, rather than ones made of multiple bone fragments (Lahti 2019). The medieval decoration of the skull relic wrappings relating to the cult of 11,000 virgins is often limited to small-scale embroidery using flowers and letters with pressed metal discs or sequins (Arponen et al. 2018b). In contrast to this, the narrative embroidery in the wrapping of the Turku Cathedral relic seems to be unique (Lahti 2019). However, Lahti (2019) suggested that the relic could have been a representation of the cult, even if not from Cologne. Lahti also mentions that there are at least two similar objects to the relic in the Museum of Cologne.
The composite skull shaped relic in Turku Cathedral is clearly smaller than a typical adult human skull. Its dimensions are 192mm (length) × 141mm (width) × 120mm (height). The construction is made from 21 linen packages containing bones and sewn together with linen yarn. The number of bones per package varies from one to over 30. Most of them are human skull bones. The 14C dating results suggest that the remains are from at least eleven different individuals (Arponen et al. 2018b). The skull construction is covered with one linen and two silk cloths, the outermost of which is a red Chinese damask with mythological characters and an embroidery depicting the martyrdom of a saint. The silk material is very rare in Nordic countries, and this is one of the earliest fragments discovered in Europe. The silk cover suggests that the skull relic was special and high status in relation to other relics (Lahti 2019).
The 14C dating results on the bones cover a time span of around 1500 years, from the 4th or 3rd century BC to the 14th century AD. The dating results of the youngest textiles used in the wrapping determine the terminus post quem for the assembly of the relic to the first half of the 14th century AD. The terminus ante quem, on the other hand, is determined by the knight's costume depicted in the embroidery: it represents a fashion that was already passé by around AD 1350. Hence, it is reasonable to assume that the relic was assembled at some point in the second quarter of the 14th century AD (Arponen et al. 2018b).
If the bones in the composition of the relic were sourced collectively, it may be inferred that they were procured from a cemetery (or cemeteries) whose use may have spanned several centuries. Moreover, such a cemetery would need to have contained inhumations from which it was relatively easy to access the skulls of interred individuals. Such cemeteries existed widely in the Roman world after the spread of Christianity began to influence burial customs. As the assembly of the skull relic took place in the second quarter of the 14th century, it is tempting to connect the origin of the bones to Avignon and the Papal Curia, which was visited by Bishop Hemming of Turku in the 1340s (Klockars 1960, 151–78). In Avignon, the cemetery by the Abbey of St Ruf was already in use by the 5th or 6th century AD and burial continued – albeit probably with a long hiatus – up until the High Middle Ages (Hartmann-Virnich 2011, 324–25). In Arles, the famous cemetery of Alyscamps was in use from the Roman period up until the High Middle Ages (Heijmans 2013). In Marseilles, inhumation burials of Greek origin have been found, hence bones older than the 1st century AD were also available (Newman and Michel 2013). Ideally, more information about where this relic could originate from would be helpful, and if the isotope signal suggests any of these well-known locations.
The use of strontium isotope analysis is widely used in migration studies (e.g. Bentley 2006; Montgomery 2010; Frei 2012; Oras et al. 2016). During mineralisation, strontium replaces calcium in bone and plants, meaning most animal tissues and plants will yield measurable quantities (see Burger and Lichtscheidl 2019 and references therein). This, combined with the variability evident in strontium isotope ratios from different locations in the biosphere, means that strontium isotope analysis has potential to infer an individual's place of origin. Strontium isotope composition of rocks varies because, while strontium-86 (86Sr) is stable, strontium-87 (87Sr) will vary. 87Sr is a product of the decay of rubidium-87 (87Rb), which has a very long half-life (48.8 billion years). This leads to age-dependent strontium isotope ratio (87Sr/86Sr) variation in geological formations (Bentley 2006) and in the associated biosphere. If there is sufficient bedrock age variability across or between geographic regions, strontium isotopes can be used to assess mobility or source locations. It is assumed that the strontium isotope composition of analysed bones and textiles reflects the underlying geology and biologically available strontium isotope composition of the biosphere from where the plant(s) used for the textiles grew.
Diagenesis is a process that takes place post-burial. It has been noted that the bone minerals can exchange strontium from the environment, which can lead to alteration of biogenic strontium isotope ratio values (Nelson et al. 1986). More specifically, the determined isotope ratio value no longer reflects the incorporated strontium during the life of the individual, but instead that of the burial environment. Bone material is more susceptible to alteration than enamel and not often used for this reason. However, in this study the strontium isotope composition is interesting regardless of whether it reflects the burial environment or the original 'living human' geographical variation. Because these bone fragments are in secondary use, it would be highly valuable knowledge if this points into a single location or if the strontium isotope composition is varied, pointing to different geological areas (of either burial environment or the person itself). Thus, it was decided not to remove anything from the sample. It is acknowledged that it is impossible to estimate the rate of secondary strontium in the analysed bones, but this is considered in Section 7.
Textiles analysed in this study do not indicate burial or the use of dyes. They are all in good condition, thus this question of diagenetic alteration is only relevant with regard to the bone samples. However, I used a protocol shown to be effective at removing dyes without further effects on strontium isotope composition (Frei et al. 2010).
Bone apatite has oxygen compounds which have been shown to originate from drinking water (Longinelli 1984). Drinking water, if meteoric, directly reflects the precipitation of the site from which it was collected, thus making it possible to characterise the climate of the geographical area where an individual lived (Daux et al. 2008). However, oxygen isotope values fractionate during each chemical reaction that they are subjected too, such as cooking, or mineralisation of bone, leading to a new value. Moreover, the variability of these chemical reactions within each individual human body, coupled with small variations between drinking sources at a localised level, can cause intrapopulation variability, typically within 2% variance (Lightfoot and O'Connell 2016). The measured values are calibrated using a formula to estimate the original 'drinking water' values because of the fractionation of the light isotopes (Daux et al. 2008; Chenery et al. 2012). These drinking water values vary geographically and reflect the origin of the water. This makes the oxygen isotope values useful and, together with the strontium isotope values and other kinds of evidence, can help in tracing the regional origin of the bones used in the Turku relic.
Bone is living tissue. The maintenance involves slow replacement of old bone with new. This is known as remodelling (see Clarke 2008 and reference therein). Bone remodelling is a continuous process that includes the replacement of the mineral component of bone, bioapatite. The bioapatite has carbonate in its structure. The carbonate is a source of carbon and oxygen analysed in this study. Because of remodelling, the oxygen isotope composition of bone material reflects a long period of time, covering decades. Bone apatite oxygen isotope composition is also sensitive to diagenetic alteration (Nelson et al. 1986). Like the strontium results, I do not believe these bone fragments to have been buried in Finland and the results are interesting regardless of whether they reflect the burial environment or the original signal.
Five bone fragments (lp2, lp6, lp8, lp13 and lp15) in the Turku relic were sampled with a hand-held diamond-tipped drill-bit. A duplicate bone sample was taken from lp8, Ip8A and Ip8B. Each bone sample was taken from the edge of the bone and size was kept to a minimum (aiming at 10mg). Further, fabric samples were taken from either the warp or the weft of five different pieces of the linen fabric. From two pieces of linen, lp6-f and lp13-f, both the warp (A) and the weft (B) were sampled (lp6A-f, lp6B-f, lp13A-f, and lp13B-f) because of possible different origins.
The bone samples were purified for strontium isotope analysis with column chemistry using the strontium specific resin method (see Charlier et al. 2006). Bone samples were ground using an agate mortar and pestle. Enamel powder was collected using a dental drill. Purified Sr was collected using 3% HNO3. All acids used for chemistry and analysis were distilled to remove contaminants. Purified Sr samples were analysed at the Department of Earth Sciences at Durham University using a Thermo Fisher Neptune plasma sourced multi-collector inductively coupled mass spectrometer (MC-ICP-MS) using inter-laboratory and international standards (NBS987 0.710250 ± 0.000013, n=11, 2σ). Rubidium and krypton were actively monitored during the analysis. Precision of individual runs of 0.00021 (2SD) or higher was achieved. Blanks were not used because the level of strontium in the bone material greatly exceeded the possible contamination in the distilled acids used in the analysis.
The fabric samples were pre-treated using methods described in detail in Frei (2014). Samples were washed using 20% HF in an ultrasonic bath and rinsed using MilliQ water. Then the sample was washed using 1N HCl and rinsed again with MilliQ water and dried. The sample was washed for a third time using 0.2M (NH4)2S2O8 and rinsed with water and dried. The samples and their pre-treatment dilutions were analysed for quality control. The remaining fabric was dissolved in 1:1 mixture 30% HNO3 and 30% H2O2 and dried in a hot plate. Samples were taken up using 3N HNO3 and loaded on disposable pipette tip columns containing Sr specific resin. The Sr fraction was eluted using water and dried. Dried samples were dissolved using 2.5 µl Ta2O5-H3PO4-HF and loaded on filaments. All procedures were done in a clean laboratory facility. The fabric samples and dilutions were analysed at the Department of Geology at the University of Copenhagen using Sector 54-IT thermal ionisation mass spectrometer (TIMS) with eight Faraday detectors. The instrument was monitored using an international standard (NBS987 0.710239 ± 0.000018, n = 4, 2σ). All results were corrected according to Thirlwall (1991). Rubidium and krypton were actively monitored during the analysis. Precision of individual runs of 0.0006 (2SD) or higher was achieved. Fractionation of strontium isotope composition is smaller than that which can be detected using regular instruments, and fractionation during analysis is eliminated using internationally agreed protocols (Halicz et al. 2008).
The bone samples taken for oxygen analysis were surface-cleaned with a hand-held drill and powdered using an agate pestle and mortar. The sample was placed in a micro-tube and 1.8ml of 1% NaOCl solution was added. The sample was rinsed with MilliQ water and pre-treated with 0.1M acetic acid to remove mineral particles. Samples were freeze dried. The oxygen isotope analysis was carried out at the University of Bradford using an isotopic ratio mass spectrometer (IRMS) instrument. International (CO-8 and NBX19) and intra-laboratory references were used and a precision of >0.2 per mille was obtained.
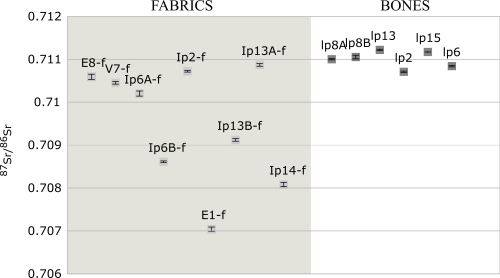
Results of the 87Sr/86Sr ratios are presented in Table 1 (see Figure 3). The strontium isotope composition in bone samples varied from 0.71071 to 0.71122 (mean 0.71010) and in the fabrics from 0.70704 to 0.71087 (mean 0.7095). The strontium isotope composition of the fabrics has a much larger range than that of the bones. Moreover, all fabric samples, and only two bone samples, have strontium isotope ratios less than 0.711.
Results from the textile analysis are reported in Table 2. During textile analysis, all acids used were also measured for strontium isotope ratios. The use of hydrofluoric acid (HF) aims to remove any dust or mineral particles from the fabrics (Frei et al. 2010). This is also seen as higher strontium isotope ratio, and it is likely that the samples had some dust after 700 years in the church environment. A second wash using the hydrochloric acid (HCl) removes carbonates to make sure there is no secondary strontium. Ammonium peroxydisulfate (APDS) was used to remove dye (Frei et al. 2010).
| Sample | Type | 87Sr/86Sr | 2 SE (abs) |
|---|---|---|---|
| E8-f | plant fibre | 0.71059 | 0.000060 |
| V7-f | plant fibre | 0.71045 | 0.000030 |
| Ip6A-f | plant fibre | 0.71020 | 0.000060 |
| Ip13A-f | plant fibre | 0.71087 | 0.000030 |
| Ip13B-f | plant fibre | 0.70912 | 0.000030 |
| E1-f | plant fibre | 0.70704 | 0.000050 |
| Ip2-f | plant fibre | 0.71072 | 0.000020 |
| Ip6B-f | plant fibre | 0.70861 | 0.000020 |
| Ip14-f | plant fibre | 0.70808 | 0.000050 |
| lp 8 A | bone | 0.711002 | 0.000016 |
| lp 8 B | bone | 0.711054 | 0.000042 |
| lp 13 | bone | 0.711220 | 0.000016 |
| lp 2 | bone | 0.710709 | 0.000015 |
| lp 15 | bone | 0.711172 | 0.000017 |
| lp 6 | bone | 0.710841 | 0.000017 |
| Sample no. | Pre-treatment | Description 87Sr/86Sr | 2 SE (abs) | |
|---|---|---|---|---|
| E 8 | HCl leach | plant fibre thread | 0.711 | 0.00001 |
| E 8 | HF leach | plant fibre thread | 0.72438 | 0.00003 |
| E 8 | APDS leach | plant fibre thread | 0.72153 | 0.00002 |
| E 8 | residue | plant fibre thread | 0.71059 | 0.00006 |
| V7 | HCl leach | plant fibre thread | 0.71046 | 0.00001 |
| V7 | HF leach | plant fibre thread | 0.72842 | 0.00004 |
| V7 | APDS leach | plant fibre thread | 0.70751 | 0.00001 |
| V7 | residue | plant fibre thread | 0.71045 | 0.00003 |
| Ip6A | HCl leach | plant fibre thread | 0.70996 | 0.00001 |
| Ip6A | HF leach | plant fibre thread | 0.71857 | 0.00004 |
| Ip6A | APDS leach | plant fibre thread | 0.7096 | 0.00001 |
| Ip6A | residue | plant fibre thread | 0.7102 | 0.00006 |
| Ip13A | HCl leach | plant fibre thread | 0.71015 | 0.00001 |
| Ip13A | HF leach | plant fibre thread | 0.71605 | 0.00006 |
| Ip13A | APDS leach | plant fibre thread | 0.71421 | 0.00001 |
| Ip13A | residue | plant fibre thread | 0.71087 | 0.00003 |
| Ip13B | HCl leach | plant fibre thread | 0.71014 | 0.00001 |
| Ip13B | HF leach | plant fibre thread | 0.71664 | 0.00005 |
| Ip13B | APDS leach | plant fibre thread | 0.71427 | 0.00005 |
| Ip13B | residue | plant fibre thread | 0.70912 | 0.00003 |
| E1 | HCl leach | plant fibre thread | 0.71038 | 0.00001 |
| E1 | HF leach | plant fibre thread | 0.71378 | 0.00004 |
| E1 | APDS leach | plant fibre thread | 0.70825 | 0.00004 |
| E1 | residue | plant fibre thread | 0.70704 | 0.00005 |
| Ip2 | HCl leach | plant fibre thread | 0.7119 | 0.00001 |
| Ip2 | HF leach | plant fibre thread | 0.73136 | 0.00004 |
| Ip2 | APDS leach | plant fibre thread | 0.70982 | 0.00006 |
| Ip2 | residue | plant fibre thread | 0.71072 | 0.00002 |
| Ip6B | HCl leach | plant fibre thread | 0.70978 | 0.00001 |
| Ip6B | HF leach | plant fibre thread | 0.71566 | 0.00005 |
| Ip6B | APDS leach | plant fibre thread | 0.70834 | 0.00007 |
| Ip6B | residue | plant fibre thread | 0.70861 | 0.00002 |
| Ip14 | HCl leach | plant fibre thread | 0.71024 | 0.00001 |
| Ip14 | HF leach | plant fibre thread | 0.71679 | 0.00006 |
| Ip14 | APDS leach | plant fibre thread | 0.71374 | 0.00006 |
| Ip14 | residue | plant fibre thread | 0.70808 | 0.00005 |
The oxygen δ18OVSMOW values of carbonate vary between -22.6 and -24.9 ‰ (see Table 3 and Figure 4). The results were converted to drinking water values using Chenery et al. 2012 conversion formulas. The oxygen isotope ratio of drinking water values (δ18ODW using Chenery et al. 2012 and Daux equation 4 and 6) varies between -10.2 and -13.7‰. The difference between conversions using the two mentioned formulas was between 0.6 and 0.9‰. The Daux formula 4 is largely dominated by their Greenland case study, while formula 6, in contrast, is based on data from several southern locations (the south of France). Because Finland is situated in a northern location, but the origin of the material is unknown, it is important to see the results using both formulas.
| δ13C VPDB ‰ | Std dev. | δ18OVSMOW ‰ | Std dev. | % CO3 | δ18OP ‰ | VSMOW Chenery et al. | Daux et al. 4 | Daux et al. 6 |
|---|---|---|---|---|---|---|---|---|
| -12.7 | 0.05 | 24.2 | 0.09 | 6.7 | 35.0 | 34.9 | -10.7 | -10.1 |
| -12.7 | 0.06 | 24.3 | 0.11 | 6.2 | 35.0 | 35.0 | -10.7 | -10.0 |
| -13.4 | 0.12 | 22.6 | 0.12 | 3.3 | 31.9 | 31.9 | -13.7 | -12.8 |
| -13.2 | 0.10 | 23.6 | 0.17 | 1.8 | 33.8 | 33.8 | -11.8 | -11.1 |
| -11.9 | 0.03 | 24.5 | 0.16 | 6.9 | 35.5 | 35.5 | -10.2 | -9.6 |
| -14.3 | 0.07 | 24.2 | 0.15 | 3.7 | 35.0 | 35.0 | -10.7 | -10.1 |
Typically, strontium isotope composition is analysed from enamel, which is one of the most taphonomically resistant of human materials. When dealing with bones and textiles, there is always a risk of contamination and alteration from the archaeological context (i.e. if the sampled materials have been in contact with water or diagenetic sediments). This is because strontium is water soluble. Thus, any archaeological material in a burial context is likely to exchange strontium isotope composition with the surrounding soil and water. However, the studied fabrics have never been buried, thus contamination owing to local water or soil chemistry is unlikely. The soil remains in the bone fragments, in turn, indicate that at least some of these were buried before the relic was assembled.
The linen fabrics in the relic are an off-white colour and were probably never dyed. Even if dyes were used, the pre-treatment method has been shown to remove dyes effectively enough to enable the measurement of the strontium isotope signal of the fabric (Frei 2013). The results very likely present the 'original' isotope signal, reflecting the conditions of when/where the fabrics were made.
Currently no strontium isotope human data is available in the Turku region, and as a result local values have to be inferred from geological data. The main bedrock in Finland is old crystalline stone. In general, old granites typically have 87Sr/86Sr ratios between 0.710 and 0.740 (Bentley 2006). This is also likely to be the case in Finland (Kaislaniemi 2011). Even though currently there are only a small number of analyses available from Finland, the estimation derived from the Finnish bedrock varies between 0.710 and 0.840, with most values above 0.720 (Kaislaniemi 2011). The River Aura, the main river running through Turku, has an isotope composition of 0.7320 (Löfvendahl et al. 1990). This composition probably reflects the geology of the surrounding area (Kaislaniemi 2011), thus indicating that the values around the Turku region are much higher than the values measured in the materials (bone and fabric) used to assemble the relic. Hence, it is unlikely that the bones and the fabrics in the relic were from Turku or the nearby vicinity. Additionally, there are no signs of burning on any bones, although cremation was the favoured burial practice in south-west Finland up until the 6th century AD, and half of the bones pre-date the 6th century, further suggesting that the bone material used was imported into the country (Figure 2; Hiekkanen 2010). Further studies are needed to show the biologically available strontium isotope composition, but with the current knowledge there are no indications of such values in Finland.
The range of strontium isotope composition derived from the fabrics is much larger than that of the bones. This might reflect their scattered origin. None of the results compare favourably with the composition of the local Finnish bedrock but, rather, indicate a match with an area of much younger geology. In Northern Europe, such sediments and bedrocks exist in Denmark and in the Baltic countries. However, there are alternative viable possibilities from across much of Europe (see for example Voerkelius et al. 2010 and Figure 4). None of the fabrics indicate a Finnish origin – not even the simple tabby woven linens, which could be considered as everyday fabrics for the time (Vajanto 2013). These preliminary results all indicate that all the materials used in the Turku relic originate from outside of Finland. Whether or not the materials were imported to Turku at the same time is still unknown.
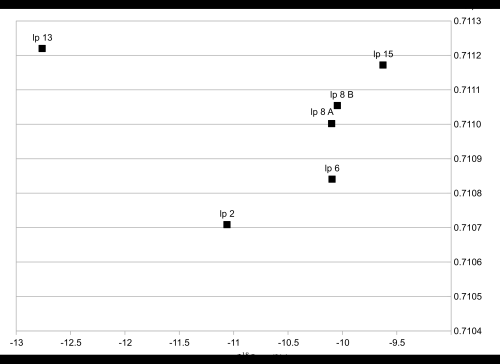
By using the Chenery et al. (2012) and Daux et al. (2008) no. 6 conversion formulas, the ratios of the drinking water oxygen isotope (δ18ODW) composition is between -9.5 and -12.7‰ (see Figures 4 and 5). Taking into account the variability of the inherent conversion formulas (at least 0.9‰), the analytical uncertainty (0.2‰), and comparing the analysed range with variability expected within a typical population (2 permille points), the possibility that the sampled bones originate from one population cannot be excluded. This also shows that the oxygen isotope composition alone is not a sufficiently precise method to study migration, but it does give a rough indication of the region of origin. It is also possible that the oxygen isotope composition is affected by diagenetic processes and indicates the place of burial rather than the place of origin. Moreover, the conversion formulas are designed for enamel and further studies should establish if bone materials reflect the geographical origins as well.
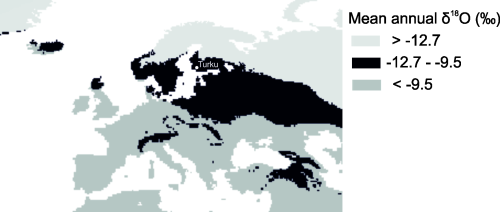
Typically, this δ18ODW value range (from -13 to -9 ‰) has been observed widely in Northern Europe, in Denmark, Sweden and the Baltic countries (Bowen and Wilkinson 2002), and in the Alpine region. For example, rivers in Switzerland, have given oxygen delta values from -14 up to -10‰, (Schürch et al. 2003). The annual mean oxygen isotope composition in Southern Finland varies seasonally between -15.0‰ and -6.5‰ and having a two-year mean value of -11.6‰ (Kortelainen 2007). Although this does not preclude the Turku area as a possible origin, together with the strontium results it seems much more likely that the constituent materials of the relic, both the fabric and osseous material, originate from outside of Finland. It is not possible to exclude that this value is being altered by burial processes and does not reflect the origin of people in question.
The date of the relic bones reflect the fact that these are not likely to be from one person and thus cannot represent a single person. The first assumption regarding the intended identity of the Turku relic was that it pertained to the patron saint of Finland, St Henry. According to the legend, St Henry was an English-born bishop of Uppsala. The strontium isotope ratios measured in this study fall within the range discovered in Britain (0.7078-07165; Evans et al. 2010). However, in Britain, mean drinking-water δ18Ovsmow values range from -9.0‰ to -5.0‰ (Darling et al. 2003). Samples from archaeological human remains in the UK provide apatite δ18Ovsmow values with a variation of 17.7 ± 1.4 ‰ (2SD) (Evans et al. 2012). In the analysis, values of δ18Ovsmow are higher than those found in Britain (these results from 22.6 to 24.9 ‰) and converted to drinking water values lower than those in Britain (-5.0‰ to <8.5‰, these results -9.5 to -13.7‰), making it unlikely that the bones in the relic would have originated from the British Isles. As shown in Figure 5, the modern mean annual precipitation δ18Owsmow in the northernmost part of Scotland would fall within the range of some of the bones used in the relic, but this area is particularly bad for bone preservation of the sort present in the remains used for the relic's construction. While similar to Finland, the typical soils are acidic and bones do not survive for long when buried (Ukkonen 1993).
The strontium and oxygen isotopic signature in bones reflects only the final antemortem period, assuming no alteration during burial processes. Thus, if the individual had stayed in one place long enough prior to their death, it may not be possible to infer an extra-local place of origin, as they would give a signal that is indistinguishable from other members of the local population. According to the legend, Henry stayed in Uppsala, where strontium values can be expected to be as high as in Finland (Price et al. 2013), thus making it unlikely that the bones in the relic originated from that region. Equally, the bones cannot be interpreted as representing those of St Eric who also lived in Uppsala region. That said, however, bone strontium composition provides a mean value reflective of a relatively long period of time, and if the saint's time in Uppsala was short-lived, it would not give comparably local values.
As 14C dates already suggest, this cannot be one individual. Thus, it could be possible that it has been composed in one of those areas associated with the saints using available human bone material. Therefore, it is an interesting point that the isotope values do not suggest these areas.
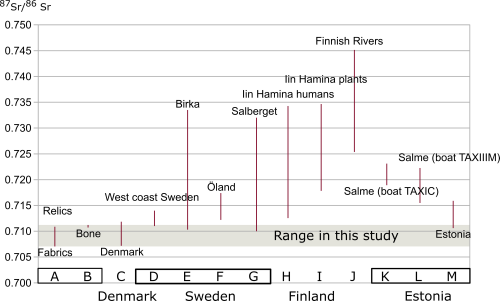
The strontium isotope compositions in fabrics and bones are similar to those found in Denmark and the south of Sweden. They are actually very similar to several locations in Europe outside Finland (Hoogewerff et al. 2019), such as parts of Germany, UK, Italy, Hungary, Romania or Spain. However, the oxygen isotope ratios indicate an area with values similar to those found in north or north-eastern Europe today and high latitudes, suggesting that the relic originated either from Northern Europe (such as southern Scandinavia or the Baltic region) or the Alpine region.
The range of strontium isotope composition exhibited in the studied bones is similar to the range measured within a single geological formation in Britain (variation within the third decimal, Evans et al. 2012). This is not proof positive, but it is possible that the bones in the relic originated from a single geological environment or that they could have been buried in a single location. Although it is not possible to determine the exact origin, the isotope measurements suggest that the place of origin could be in southern Scandinavia, Baltic countries or somewhere further south, while remaining in northern Europe. The bones were probably sourced from a single geologically homogenised and discrete geographical area. These results should be also tested using other methods and when new research using other sources update, the results can be reanalysed. Because of similar oxygen values in the Alpine region, the results presented here do not necessarily preclude southern France as the place of origin for the bones.
More research is needed in both use of the methodology of such pre-treatments, and in materials to fully study the suitability of the protocol with linen. It would also be vital to get better understanding of strontium isotope variation in the north Europe which would help to develop the interpretation presented here These results are preliminary and I hope will be further amplified by larger sampling and comparative materials.
Both the oxygen and strontium isotope composition of the Turku relic bones and fabrics provide new information regarding the provenance of the analysed materials. I suggest that the relic was likely imported into the country. While it is not possible to determine a single location as a place of origin, all the isotope composition values suggest that the various materials used were all sourced from elsewhere in northern Europe, or the Alpine region, but outside of Finland. Moreover, both oxygen and strontium isotope data would be similar to values found in areas like south Sweden, Denmark or the Baltic countries but more research needs to be conducted to find the place of origin. The date range of the osseous material points to a location where there must have been either a large collection of bones, or where inhumation would have continued at least intermittently throughout the first millennium AD.
More research on these relics and strontium or oxygen isotope methodology, especially for linen, is needed. This preliminary study can only reveal that the artefact is likely to have been made outside Finland. Similar geological areas occur in various parts of Europe and with this study it is not possible to find an exact place of origin. However, such a place of origin could not be established in any of the suggested areas for St Henry, St Eric or St Ursula.
I would like to thank sincerely Professor Karin Frei for help in fabric strontium analysis and guidance on laboratory work, Professor Robert Frei for running the analysis, Dr Geoff Nowell at Durham University for allowing the use of their laboratory and help with running the samples, and Dr Julia Beaumont for allowing oxygen analysis at the University of Bradford. I would also like to thank conservator MA Aki Arponen for high-quality discussion on relics and help with background literature. I would also like to thank Professor Jussi-Pekka Taavitsainen, the Head of the Turku Cathedral Relic Project, for the initial idea of the study. I would like to thank the Finnish-Danish Cultural Foundation and the Relic Project for funding. The author is solely responsible for the content of the article. Finally, I would like to thank the anonymous reviewers whose suggestions significantly improved the quality of this study.
Internet Archaeology is an open access journal based in the Department of Archaeology, University of York. Except where otherwise noted, content from this work may be used under the terms of the Creative Commons Attribution 3.0 (CC BY) Unported licence, which permits unrestricted use, distribution, and reproduction in any medium, provided that attribution to the author(s), the title of the work, the Internet Archaeology journal and the relevant URL/DOI are given.
Terms and Conditions | Legal Statements | Privacy Policy | Cookies Policy | Citing Internet Archaeology
Internet Archaeology content is preserved for the long term with the Archaeology Data Service. Help sustain and support open access publication by donating to our Open Access Archaeology Fund.