The different compositional signatures between the Ramesside (19th-20th Dynasty) and 18th Dynasty glass discussed above has been used to support the premise that a different cobalt source was used in the later period at Dahshur (Abe et al. 2012), predominantly because the Al2O3 levels in the Ramesside glass were significantly lower than the empirical limit of 1.2%Al2O3 found in most cobalt glasses. Furthermore, and more significantly, the differences in alumina levels have been used to suggest that glass production continued after the New Kingdom period, i.e. a new cobalt source was exploited for this later glass.

The plots (Figures 13-14) show the distribution of CoO, NiO, MnO and ZnO in the 18th Dynasty glass plotted in Figure 6. Figure 13 shows that the distribution of cobalt oxide concentration in the 18th Dynasty glass is bimodal, with two distinct peaks around 0.07 and 0.16% cobalt, i.e. the lower peak having about half the CoO level of the higher peak. Both NiO and MnO show similar bimodal distributions (Figure 14). Furthermore, as with CoO, the peaks at lower concentrations for both NiO and MnO appear to be about a factor of 2 lower than the peaks at higher concentrations. Although more difficult to discern, Al2O3 and ZnO exhibit broad distributions commensurate with more than one peak. This suggests that the lower concentration peaks could simply represent a dilution of the higher concentration peaks, i.e. cobalt glass with 0.16wt% cobalt was mixed with a base glass (containing only trace levels of CoO, NiO, MnO and ZnO) in approximately a 1:1 ratio. ZnO is consistent with this interpretation, with its broader distribution perhaps reflecting that it can be reduced when heated to 950°C (Greenwood and Earnshaw 1997) in the presence of carbon, resulting in the volatile Zn metal. The lower concentration peak of the Al2O3 distribution, however, is less than a factor of 2 lower (2.8%) than the upper peak (3.9%), which could reflect additional grinding with the base glass before dilution resulting in further contamination, the use of a silica source with higher alumina levels for the base glass, or contamination from the crucible during re-melting. Again, regardless of the mechanism, the data are consistent with the proposition that the 18th Dynasty glass had an initial average composition of around 0.16%CoO, 0.11%NiO, 0.38%MnO and about 0.15%ZnO and 3.9%Al2O3 (i.e. the higher peaks) prior to any dilution. Some of this composition of glass would have been used directly to make objects, while some was re-melted, potentially a consequence of recycling broken objects. In effect, these distributions would suggest that some of the cobalt-blue glass was re-melted during the 18th Dynasty.
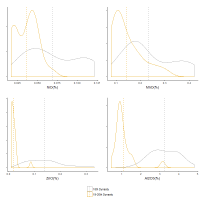
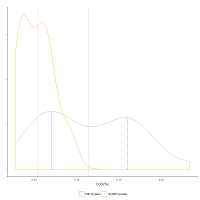
What is also apparent from the density plots is that the distribution of the glass from the Ramesside period at Dahshur is lower in concentration for all these oxides compared to the 18th Dynasty glass. Although it is difficult to state with certainty whether this is a consequence of further dilution of existing 18th Dynasty glass, this interpretation is supported to some degree by the average compositions (dashed vertical lines in Figure 13 and Figure 14) for each oxide during both periods, i.e. CoO, NiO and MnO potentially show further dilution by a factor of about 2 for this later glass. In other words, the difference in the average concentrations could be interpreted as glass from both compositional distributions from the 18th Dynasty being further diluted with a base glass. This would suggest that the Ramesside (19th-20th Dynasty) glass from Dahshur in Figure 11 derives from glass that originally fell on the granite line with the 18th Dynasty glass, before subsequently shifting to lower levels of alumina after dilution. If this is the case, then it would indicate that the Ramesside blue glass was not made with cobalt from a different source but derived from re-melting existing 18th Dynasty glass. This proposition is now further examined.
The dilution practice of re-melting 18th Dynasty glass in the 19th-20th Dynasties is to some degree revealed by examining the alumina and titania distributions. As noted above, the lower peak of the Al2O3 distribution for the 18th Dynasty glass is less than a factor of 2 lower (2.8%) than the upper peak (3.9%) (Figure 14). Furthermore, the average Al2O3 level of the whole 18th Dynasty distribution is more than twice as high as the average of the 19th-20th Dynasty glass (3.2% and 1.05%Al2O3, respectively). This suggests that the lower peak of the 18th Dynasty glass (and thereby the average level of the all the 18th Dynasty glass) is higher than expected from the first 1:1 dilution with a base glass. In other words, the higher alumina level is potentially due to contamination from the furnace lining or crucible during recycling, which would increase both the alumina and titania concentrations (but not the ZnO, NiO, MnO or CoO concentrations), i.e. Nile silt has average Al2O3 and TiO2 concentrations of about 15% and 2%, respectively (Shortland et al. 2006b). This glass was diluted again to produce the 19th-20th Dynasties glass. The range of the TiO2/Al2O3 ratio for both Nile silt (Shortland 2000), Ballas clay (from the edge of the Western Desert near Luxor) (Baba 2009) and pottery found at Amarna and Malkata (Shortland et al. 2006b) are plotted in Figure 15, which shows that decreasing cobalt oxide levels (i.e. more dilution) results in an increase in the TiO2/Al2O3 ratio approaching a level consistent with materials used for the furnaces and crucibles. Figure 16 shows that the TiO2/Al2O3 distribution is much narrower for high cobalt concentrations (i.e. 18th Dynasty glass) but covers a wider range at lower CoO concentrations resulting in a much wider distribution for the later Ramesside period glass, i.e. more mixing results in more contamination and, therefore, more variation.
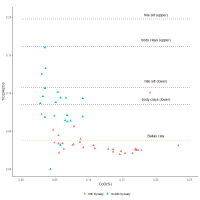
In essence, Figures 15 and 16 suggest that alumina and titania, derived from minerals found in the furnace or crucible materials (e.g. feldspar, rutile etc.), contaminated the 18th Dynasty glass, raising the alumina and titania levels to higher than expected from the first dilution, before subsequent dilution again in the Ramesside period. This is supported by the fact that alumina and titania are less well associated with further dilution (i.e. recycling) as evidenced by the differences in the dendrograms for the 18th Dynasty and 19th-20th Dynasty glass in Figure 8 and Figure 9. In other words, alumina and titania initially entered the glass system together but contamination from re-melting and reworking affected the strength of this association.
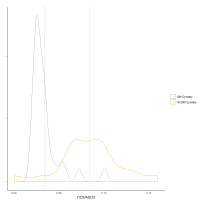
Further support for mixing is found by examining possible contamination from lead. Recycling coloured glass requires that colours are separated prior to re-melting. This is unlikely to be a completely efficient process, with elements required to make other colours potentially contaminating the cobalt-blue glass. Although an indirect measure, the fact that the 18th Dynasty glass from the museum collections has an average of 0.021%PbO while 19th-20th Dynasty glass from Dahshur has an average of 0.041%PbO strongly suggests that yellow glass (coloured by lead antimonate) entered the system. Furthermore, PbO is not well associated with the other components, clustering only with copper (Figure 8 and Figure 9). The distribution (Figure 17) shows that most of the 18th Dynasty glass has low levels of PbO, which could potentially be the trace levels prior to any recycling (the peak in Figure 17 is about 0.01%PbO). Some 18th Dynasty glass has elevated levels of PbO, providing further support that recycling occurred during this period. The 19th-20th Dynasty glass has a much broader distribution than the 18th Dynasty glass, suggesting that the majority of this glass has been contaminated with lead, which is consistent with recycled glass. The weak association of copper with the other components (Figure 8 and Figure 9) is also indicative of contamination, from copper-blue glasses. Interestingly, Sb2O3 clusters with the other components rather than with PbO (Figure 8 and Figure 9). This is possibly a consequence of contamination from white opaque glass, where antimony is associated with calcium thereby weakening its association with lead, i.e. calcium antimonate (white glass) and lead antimonate (yellow glass) both entered the system during the recycling process.
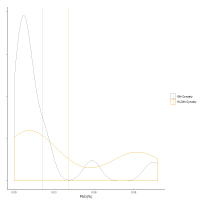
In effect, the initial 18th Dynasty glass (the higher peak concentrations of CoO, NiO, MnO and Al2O3 in Figure 13 and Figure 14) was diluted at least twice (by a factor of around 4) by the Ramesside period. From the distributions in Figure 13, Figure 14, Figure 16 and Figure 17 it is also possible to infer that more than one dilution occurred during the Ramesside period. Although such dilutions were probably derived empirically, it is interesting to note that the cobalt level in the Ramesside glass (average CoO = 533ppm) was around the lower limit to maintain a rich, deep blue (Henderson 2000). This fits well with the narrative that glass was not produced in significant amounts after c. 1250 BC, with glass-makers reducing the levels of cobalt through dilution with a base glass to the point where the colour was still effective.
This mixing mechanism means that there is no need to propose a new cobalt source during the Ramesside period to explain the different compositional signatures, nor to require the production of cobalt-blue glass to continue after the New Kingdom period. Furthermore, the relationship between TiO2 and Al2O3, in similar proportions to those found in igneous rocks, is sufficient to suggest that the alum mines in Egypt's Western Oases were not the source of cobalt for either the 18th Dynasty or 19th-20th Dynasty cobalt-blue glass. The repercussions regarding the provenance of cobalt glass will be discussed below. However, this proposition immediately resolves one archaeological issue surrounding the presence of cobalt in glass, in that it is no longer necessary to speculate on why such accessible sources as the Kharga and Dakhla oases, with their proximity to both Amarna and Malkata and even Dahshur (Figure 3), stopped being exploited by around 1250 BC: the claim being made here is that they had not been used for colouring glass before, and they remain unused. However, it raises several archaeological science issues regarding how to provenance the cobalt and deal with glasses that have been mixed. First, alumina can no longer be considered a reliable indicator among the suite of oxides used to inform on the cobalt source, as igneous rocks are not unique to Egypt and contamination from furnaces and crucibles are probably inevitable. Moreover, it is much more difficult to make sense of groups clustering on bivariate plots if glass has been mixed, as some points will fail to cluster in a compositional group because of dilution, irrespective of provenance. For example, from the LA-ICPMS data for the Egyptian and Mesopotamian glasses, the average ZnO level of the Nippur axes is 0.015%, which is approaching an order of magnitude lower than the Egyptian cobalt glass (ZnO = 0.114%). Although this appears to be a useful discriminator to differentiate between groups, the low ZnO values for the Nippur axes could be indicative of dilution as well as the loss of volatile zinc due to re-melting and reworking the glass. This implies that if the glass used for the Nippur axes was diluted more than once (or experienced higher temperatures or more reducing conditions during processing), the cobalt source may have been zinc-rich.
Internet Archaeology is an open access journal based in the Department of Archaeology, University of York. Except where otherwise noted, content from this work may be used under the terms of the Creative Commons Attribution 3.0 (CC BY) Unported licence, which permits unrestricted use, distribution, and reproduction in any medium, provided that attribution to the author(s), the title of the work, the Internet Archaeology journal and the relevant URL/DOI are given.
Terms and Conditions | Legal Statements | Privacy Policy | Cookies Policy | Citing Internet Archaeology
Internet Archaeology content is preserved for the long term with the Archaeology Data Service. Help sustain and support open access publication by donating to our Open Access Archaeology Fund.