The compositional signature of the cobalt-blue frit found at Amarna is probably significantly more indicative of cobalt's provenance than the composition of glass proper, which has not only been diluted but may have been contaminated during recycling. The compositional analysis of the cobalt-blue frit shows that CoO is well associated with most of the components in the frit which, apart from MnO, is generally not observed in 18th Dynasty glass (see dendrograms Figure 8 and Figure 9). This suggests that CoO derived from a siliceous rock that included Al, Ti, Mg, Co and Mn and possibly Fe and Cu. It is also probable that Ni and Zn were present, but at levels too low to be measured by the WDS technique, as observed generally in cobalt-blue glass (e.g. Figure 6). Even the low concentrations of potash appear related to cobalt, as evidenced by the possible linear interaction between K2O and CoO in Figure 21, suggesting they derive from a common source. In essence, it is these components that can be used to provenance cobalt. Furthermore, even the trace levels of P2O5, SnO2 and PbO found in some of the frit samples (Table 2) may be diagnostic regarding the source of the cobalt, despite being at the limits of detection for WDS. Moreover, the presence of sulphur found in the cobalt-blue Malkata glasses (n = 12) at an average level of 0.35%SO3 (range: 0.24-0.56%SO3) using WDS, suggests that some of the components may initially have been in the form of sulphides or sulphates (Shortland and Eremin 2006). Arsenic has been detected in Egyptian cobalt-blue glasses from Malkata (n = 12) which, despite the low concentrations and high variability, are higher than the concentrations in colourless glass (n = 7) found at the same site (colourless = 1.16ppm, s = 0.97; cobalt blue = 6.43ppm, s = 4.28) (Shortland et al. 2007). This could indicate that some of the components of the cobalt system were arsenides prior to any high-temperature processing. These components and their associations are suggestive of a five-element mineralisation in which Ni, Co and As have a high degree of correlation with each other (Kissin 1992). In the case of the Egyptian glasses, NiO and CoO are definitely well associated (Figure 2 and Figure 6). A linear relationship is also found between these components in Mesopotamian glass from Nippur using LA-ICPMS data (Figure 24, data from Walton et al. 2012).
Any association with arsenic, however, is likely to be diminished during processing as it is volatile, especially if the ores were roasted (Yin et al. 2014; Wilson and Mikhail 1989). Furthermore, arsenic losses from melt systems have been shown to increase on further re-melting, with fluctuations in temperature resulting in significant arsenic losses (Mödlinger et al. 2017). This could indicate that heating and cooling during processing (as would be expected in the working of glass into objects) as well as recycling, would result in large but unpredictable arsenic losses. For example, European cobalt-blue beads (17th century AD) were found to have arsenic levels of between 400 and 7000ppm, with concentrations potentially depending on the shape of bead produced (Hancock et al. 2000). These beads, which were provenanced to the cobalt-arsenide minerals found in the Hartz mountains of eastern Germany (i.e. high arsenic levels in the ores), not only demonstrate that low arsenic concentrations are found in cobalt-blue glasses but may also suggest that large variations in arsenic levels are related to times in the furnace (and the number of times the glass is heated and cooled) to form different types of bead, i.e. some shapes are presumably worked more than others. Section 5 investigates how arsenic levels vary on re-heating an arsenic-rich cobalt-blue glass. In essence, an absence of arsenic can still indicate a five-element ore provenance for cobalt-blue glass, a mineralisation that is also often associated with manganese, zinc, lead, copper and sulphur in a variety of host lithological environments (Kissin 1992; Bagheri et al. 2006; Tarkian et al. 1983; Mazaheri Kuhanestani et al. 2014).
Five-element deposits are found in various locations around the world such as Cobalt in Ontario, Canada, the districts of Freiberg, Jachymov and Erzgebirge in Germany (Kissin 1992) and the Anarak district in central Iran (Tarkian et al. 1983; Bagheri et al. 2006). Records regarding the exploitation of these deposits date back to the Middle Ages for Erzgebirge (Ore Mountains). The mines in the Cobalt district of Canada exploited silver and uranium in the 20th century (Kissin 1992). No records are known of when the deposits in the Anarak district of central Iran began to be exploited but the Talmessi and Meskani mines have been dormant since 1960 (Bagheri et al. 2006). This type of mineral deposit contains a distinctive suite of minerals (Co, Ni, As, Ag and ± Bi, U). However, such mineral deposits often show some variation and the entire suite is not always present. Moreover, in the Anarak district, copper arsenides also appear to be associated with this mineralisation (Bagheri et al. 2006; Mazaheri Kuhanestani et al. 2014). These deposits in central Iran are particularly relevant to the discussion here. Not only are they more likely to be associated with the glass production in Mesopotamia and Egypt – the Kashan deposit was suggested by Garner (1956) as a possible source for cobalt glass found at Eridu, Iraq c. 2300 BC - but Pb and Zn in these deposits are believed to have derived from schist while geochemical and petrography indicate that Ni, Co, Cu and As derived from ultramafic rocks (Mazaheri Kuhanestani et al. 2014). This suggests that a greater co-dependence would be expected between Ni and Co (as parts of the mineralisation), than between Zn and Co. These associations are evident in Figure 8 and Figure 9. Furthermore, the abundances of the major elements for rocks and veins from the vicinity of the Talmessi and Meskani mines in the Anarak district (Bagheri et al. 2006) have levels of SiO2 (42.65-61.55%SiO2) that are lower than the frit (84.4%SiO2), and insufficient Na2O (0.96-4.26%Na2O) for the system to be self-fluxing. In other words, SiO2 and Na2O were potentially added to extract any metal from this system, as discussed in Section 4.4.
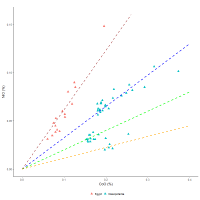
To examine this further, the linear relationship in the Egyptian 18th Dynasty glasses between NiO vs CoO (Figure 2 and Figure 6) has a gradient of about 0.6 (0.56 for the elements). Figure 24 shows the interaction using the LA-ICPMS data for cobalt-blue glass from Amarna and Malkata (from Varberg et al. 2015; 2016; Shortland et al. 2007) and Nippur (Walton et al. 2012).

In Figure 24, the gradient for the Amarna/Malkata LA-ICPMS glass is similar to the 18th Dynasty pXRF data in Figure 6, i.e. about 0.6, and passes through the origin (dark red dashed line). The glass from Nippur is more difficult to interpret. There appears to be an interaction with a gradient of about 0.3 (blue dashed line) that can pass through the origin. The Mesopotamian glass and the Egyptian glass appear to converge. There also appears to be lower groups from Nippur where it is difficult to apply linear lines.
Nonetheless, these interactions are consistent (or at least not inconsistent) with a five-element ore system that has variable levels of nickel and cobalt. The ratio of Co to Ni at Meskani and Talmessi deposits in the Anarak region of Iran range from 1:0.3 to 11:35.4 (Khoei et al. 1994; Matin 2015) i.e. Ni/Co: 0.3-3.22, respectively. This wide range would encompass both the Egyptian and Mesopotamian cobalt-blue glasses. The fraction of nickel to cobalt in skutterudite (one of the main cobaltiferous minerals in these deposits (Tarkian et al. 1983; Mazaheri Kuhanestani et al. 2014) is 0.33, i.e. empirical formula: Co0.75Ni0.25As2.5; wt%: Co = 17.95%, Ni = 5.96%, As = 76.09%). This is similar to the gradient for the Mesopotamian glass data, suggesting strongly that the cobalt in the Nippur glass was from an almost pure deposit of skutterudite. The higher gradient for the Egyptian data is potentially a consequence of variable amounts of nickel and iron replacing cobalt in this mineral, i.e. skutterudite has a variable formula ((Co, Ni, Fe) As2-3)). Less cobalt could suggest more nickel, and therefore a higher slope on the NiO vs CoO plot. However, the presence of another nickel-based mineral (such as nickeline) in association with the cobalt mineral could also increase the gradient for the Egyptian glass, potentially suggesting less-rich cobalt ore was exploited for export. Nevertheless, the ratio of Ni to Co found in both Mesopotamian and 18th Dynasty glass is consistent with minerals found in five-element deposits in Iran and provides an explanation for the high association and linear relationship between CoO with NiO. On the other hand, although ZnO is clearly associated with CoO in Egyptian glass, it is better associated with MnO (e.g. Figure 8). In essence, whereas Ni and Co are potentially part of the five-element ore mineralisation, Zn and Mn are found in associated minerals (which may or may not be present). FeO in glass is likely to have a variable concentration, owing to the iron associated with the silica used to make the base glass and is therefore difficult to relate to the ore. Figure 25, however, shows that FeO in the frit increases with CoO. Although the linearity is questionable, it is still consistent with the frit deriving iron from skutterudite, one of the main minerals in a five-element ore system.
Differentiating the components that are integral to the five-element mineralisation (e.g. Co and Ni, Fe when the main cobalt mineral is skutterudite, and Cu in the case of the Anarak deposits) from those minerals that are associated with some deposits (e.g. Mn and Zn), such as in the post-ore stage, goes some way to explaining the differences between Egyptian and Mesopotamian glass. The Nippur axes, for example, have very little manganese or zinc and the interaction between MnO and ZnO with CoO is not linear (although the absence of linearity could be a limit of detection issue). However, the linear relationship prevails between NiO and CoO (Figure 24). Similarly, the early 1st millennium Nimrud blue glasses studied by Reade et al. (2005) also do not exhibit the relationship between Mn and Co, despite being elevated in the same suite of elements as the 2nd millennium Egyptian cobalt glasses. This could be (and has been) interpreted as different cobalt minerals being exploited (e.g. asbolane vs linnaeite). However, it could also indicate the variability and the variation in the associated minerals which are found in five-element deposits. Although from a different period, seven types of cobalt-blue pigments used in Islamic ceramics and glass have been identified (Wen 2012, 249-51). These pigments, which are considered to have an Iranian provenance, have various combinations of elements (Co, Fe, Zn, Cu, Pb, As, Ni, Sb, Mn and Bi) in variable proportions, often with some elements being absent. These variations potentially reflect the Iranian cobalt ore sources exploited (see Matin and Pollard 2017).
Copper, although being well associated with the five-element ore mineralisation at mines in Anarak, is not well associated with cobalt in the frit or in glass. In glass, this lack of association is potentially due to contamination. In the case of the frit, although the compositional signature has yet to be influenced by dilution and its associated contamination from recycling, it has still undergone high-temperature processing. As with any smelting system, the absolute amounts of each component in the frit would be dependent on how each component partitions (i.e. concentrates) between the metal being extracted and the glassy slag phase. For those elements that concentrate in the metal, associations with the elements in the glass slag would be diminished. This suggests that any component that partitions primarily with the extracted metal will have lower co-dependence in the glass with those components that partition with the glass. In other words, the low levels of copper in the frit (0-0.02%) and its low association with cobalt is because some proportion of it was extracted.
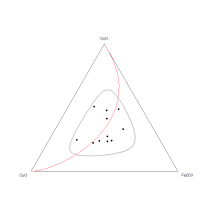
Plotting the CuO-FeO-CoO sub-composition on a ternary diagram (Figure 26) shows a broad variance ellipse. Such a broad ellipse suggests that the compositional variance is shared between each of the components in this sub-composition. In other words, although the variation is high between each component, none of these components dominates the compositional variance. This broader variance structure would be expected if the association of CuO and Fe2O3 with CoO was diminished owing to partitioning.
This also suggests that the other two components generally associated with the five-element system (i.e. Ag and Bi) may also have partitioned with the metal during the extraction process. In fact, for these metals, which are found in their native form in the five-element systems, it is quite probable that both bismuth and silver would not partition with the glass. Furthermore, any metal (or metal compound reduced to the metal) found in the glass would be in the form of entrapped prills, rather than dissolved in the glassy covalently-bonded matrix. In essence, this suggests that the extracted metal (and potentially the principal reason for processing the ore) would be absent, or present only as a rare discrete phase within the glassy slag, taking with it components that partition predominantly in the metal phase. Moreover, in terms of archaeology, considering that the cobalt frit was a by-product that was valued and traded, the metal that had been extracted may not even form part of any assemblage at the location where the frit was recovered.
There are indications that any metal removed from the system was not difficult to extract. Considering that the glass slag had value as a colourant supports the previous suggestion that the process occurred in a crucible, in order to avoid contamination. The low levels of K2O, MgO and P2O5 in the frit (Table 2) further suggest that it was produced in an environment where contamination from the products of burning wood to fire the furnace was minimised. This suggests that crucibles containing the ore minerals, the host rock and any soda-based flux additive were heated from the outside and that the main component for recovery was easily reduced, or was already in the metallic form, but embedded in the cobaltiferous siliceous rock.
As one of the main components of the five-element ore system, silver is a potential candidate for the metal that was extracted. Fahlore (a copper arsenic sulphosalt) has been found in association with significant silver and zinc contents at the Talmessi and Meskani mines in Anarak, with host rocks of dacite (an igneous volcanic rock containing plagioclase and quartz) and andesite (an intermediate between basalt and granite). Interestingly, the silver from Early Bronze Age silver objects recovered at Ur were interpreted as originating from a fahlore, objects that suggested a silver, copper, lead and zinc mineralisation and contained trace levels (<100-500ppm) of Co, As, Ni, Mn, Fe and Sn (Salzmann et al. 2016, 141-45; also see Pernicka and Bachmann (1983) for a similar interpretation at Laurion). Furthermore, of the element suite, only silver could be extracted without a strong reducing atmosphere. Cobalt, zinc, manganese, lead and nickel would require smelting with charcoal in intimate contact with the charge and, apart from lead, were not metals that were smelted deliberately in antiquity. Moreover, if copper was predominantly in the form of a sulphide, as is possible since SO3 was measured in the Malkata samples, it would be very difficult to reduce to a metal using heat alone. It was also a metal that was being readily extracted from other sources.
Silver, when in association with Ni-Co-As ores, is in the form of native silver and would therefore, not need to be reduced. Even cerargyrite (i.e. silver chloride - AgCl), would be readily reduced to silver metal. Smith (1967) argued that the earliest silver objects were made of native metal or of cerargyrite, both of which form silver on melting under a cover of charcoal. Furthermore, despite the trace levels found in the LA-ICPMS studies, silver is not always found in low concentrations in cobalt-blue glass. Figure 27 shows an energy dispersive X-ray spectrum of cobalt-blue glass found at Amarna (Nicholson and Henderson 2000). The fact that silver was detected with this analytical technique suggests that silver was present at levels greater than 0.1%, and probably higher. Moreover, it wasn't detected in the white and yellow glasses investigated. However, although silver was detected in cobalt-blue glass does not mean that it was distributed homogeneously throughout the glass matrix. As mentioned above, a metallic phase will not be soluble in glass but is more likely to be in the form of entrapped prills.
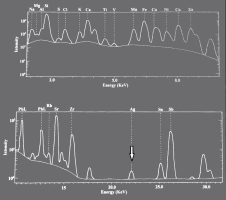
The difference in measurement techniques used by different research teams is therefore significant. LA-ICPMS is generally used to determine the composition of the glass matrix. Spot or line ablations would be constructed to avoid any particulate phases and de-spiking of LA-ICPMS data would also result in any particulate phase being smoothed out of the data in order to determine a more representative composition for the glass matrix. In essence, LA-ICPMS has been used to determine major, minor and trace elements in the matrix, while methods such as SEM-EDS, WDS and EPMA provide bulk analyses on the major components of the matrix as well as the presence of major elements in specific particulate phases, albeit at higher (i.e. worse) detection limits. This would suggest that any silver prills present in the glass slag would generally be detected only by an instrument focusing on an area, or if specific phases were being investigated. Furthermore, assuming the main reason to smelt silver-bearing ores was to extract silver, any prills observable to the naked eye (around 50 microns in diameter) would have been removed from the glass (breaking up the coloured glass frit into small transportable 'stones of casting'). Moreover, considering that the cobalt glass slag is a concentrate that would have been diluted with a base glass to produce cobalt-blue glass (probably in a 1:1 ratio) would explain not only the low amounts of silver found in cobalt glass in the archaeological record but also its high variation and low co-dependence with any other component. In essence, the EDS spectrum in Figure 27 is probably rare, as it suggests that this bulk measurement of the glass must have scanned a potentially isolated silver prill.
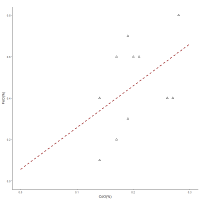
In summary, the re-analysis of the cobalt-blue frit found at Amarna suggests that it was the slag of a smelting system. The components found in the frit are consistent with a five-element ore mineralisation in which Ni and Co are intimately associated. As these systems contain native silver dispersed and embedded within a rocky matrix, it is suggested that the silver was the main reason to process these ores, with the cobalt-blue glassy slag being the by-product of the smelt. The five-element ore deposits of central Iran have the components that are found associated with the frit, suggesting that such deposits may have been exploited to extract silver and a cobalt-concentrated vitreous slag (frit). The fact that glass from both Mesopotamia and Egypt shows linear relationships between CoO and NiO and also appears to have similar host rocks (i.e. the interaction in Figure 12) could suggest that they both derived from a five-element ore system but with different post-ore stage mineralisations. In essence, although it is not the purpose here to directly link the deposits at Anarak with all cobalt-blue glass found in the archaeological record, there do appear to be some compositional similarities that may so far have been overlooked. Furthermore, the fact that cobalt glass has been found at Eridu with 0.15%CoO (Garner 1956) and at Nippur with very high cobalt levels (0.93%CoO) (von Neumann 1929), may also suggest that the use of a vitreous concentrated cobalt-blue colourant was not confined to Egypt. It could also suggest that the frit exported to Egypt had already been diluted with a base glass.
There is also indirect evidence to support the premise that cobalt-blue frit from Amarna could have derived from the Near East. In addition to Varberg et al.'s (2015; 2016) evidence of movement of glass from the Near East to Egypt and Europe, some copper blue (turquoise) glasses opacified with calcium antimonate found in Egypt have no tin. Near Eastern glass is generally tin-free (Lilyquist and Brill 1993, 71). This suggests that the Egyptian turquoise glass may have derived from the Near East before being recoloured during recycling in Egypt with bronze (perhaps after dilution), thereby introducing tin into the system. This would also provide a reason for the composition of some Egyptian blue glasses that have copper and cobalt as colourants (Smirnou and Rehren 2013), where the presence of both are clearly redundant in terms of colouring the glass. Perhaps more significantly, lead antimonate yellow early glasses found in Egypt have higher lead isotope ratios than galena ore deposits from the lead mines in Gebel Zeit in Egypt's Eastern desert and are close to the few yellow glasses found in the Near East (Shortland et al. 2000), i.e. from Tell Brak and Tell Rimah (c. 13th century BC). Lilyquist and Brill (1993, 59-65) considered that there was a Mesopotamian ore field defined by glass. Figure 28 shows LIA (lead isotope analysis) mirror plots for these glasses and for glass from Egypt (yellow glass, one red) and from Susa (yellow glass) in Iran (Lilyquist and Brill 1993), green glass frit from Egypt (19th Dynasty) (Shortland 2006), objects and litharge recovered from Sialk in central Iran (Nezafati et al. 2008, 329-50) and from Arisman (Pernicka et al. 2011), lead ores and slag from Naklak (Pernicka et al. 2011), silver from Egypt and Syria (Oxalid 2018) and the Royal Tombs at Ur (Klein et al. 2016, 89-97), copper and lead objects found at Amarna (Oxalid 2018), and ores and litharge from Iran (Stos-Gale 2001), ores specifically from the Anarak district (Pernicka et al. 2011; Nezafati et al. 2008, 329-50) and ores from Zn-Pb deposits (with sections associated with Ag) from the Urumieh-Dokhtar and Sanandaj-Sirjan zones extending from NW-SE Iran (Mirnejad et al. 2011). All archaeological samples are LBA or earlier. The 208Pb/206Pb vs 207Pb/206Pb also includes MBA silver from Sidon (Veron and Le Roux 2004) which did not have Pb204 values. See Legacy Dataset.
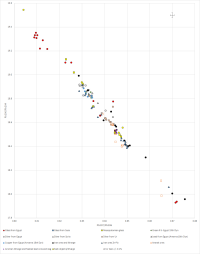
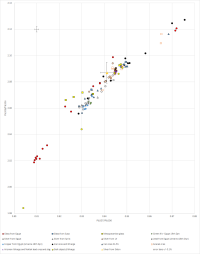
About half of the Egyptian glass samples are not only consistent with Iranian ores (i.e. Pb207/Pb206>0.82), with three being close to the ore values of the Anarak region, but some are also consistent with glass from Susa and silver from Syria, Sidon, Ur and Egypt. The eight samples closest to the Iranian ore 'field', in order of ascending Pb207/Pb206 are samples 2168 (Wadt Qirud – Tomb of foreign wives of Thutmosis III – red part – c. 1425 BC), 2169 (Assasif Tomb 37 – from a tin bead – c. 1460 BC), 2166 (Susa, c. 15th century BC), 1119 (Lisht excavations – Later New Kingdom), 2184 (Thutmosis III's tomb c. 1425 BC), 2178 (Lisht excavations – Later New Kingdom), 1118 (Lisht excavations – Later New Kingdom) and 2177 (Lisht excavations – Later New Kingdom) (Lilyquist and Brill 1993).
The issue with ore 'fields' is that they often overlap with other geographically separated areas. However, the fact that the some of the glass from Egypt is also consistent with glass from Susa, silver from Syria, Sidon, Ur and Egypt as well as ores and slag from Iran, suggests that some of the Egyptian yellow and red glass derived its isotopic signature from lead from Iran, lead found in areas with similar isotopic signatures to silver. Although the default position would suggest silver derived from an argentiferous lead ore, the possibility exists that the lead used for lead antimonate (i.e. yellow glass) was not necessarily argentiferous. In other words, lead associated with the five-element ore mineralisation would provide a similar LIA signature to the silver. These LIA data clearly support the movement of silver and yellow and red glass to Egypt as well as silver to Sidon. Green glass frit recovered in Egypt also appears to be consistent with glass from Egypt, silver from Egypt, Syria and Ur, and Iranian ores, which could suggest that copper-based frit was also produced in the Near East, especially when it is considered that the Anarak mining district has two unusually large deposits of native copper at Talmessi and Meskani (Piggot 2004, 28-43). Copper and lead objects recovered from 18th Dynasty Amarna also appear to fall close to Iranian ores, litharge and objects. It is therefore conceivable that cobalt-blue frit made a similar journey to Egypt.
Internet Archaeology is an open access journal based in the Department of Archaeology, University of York. Except where otherwise noted, content from this work may be used under the terms of the Creative Commons Attribution 3.0 (CC BY) Unported licence, which permits unrestricted use, distribution, and reproduction in any medium, provided that attribution to the author(s), the title of the work, the Internet Archaeology journal and the relevant URL/DOI are given.
Terms and Conditions | Legal Statements | Privacy Policy | Cookies Policy | Citing Internet Archaeology
Internet Archaeology content is preserved for the long term with the Archaeology Data Service. Help sustain and support open access publication by donating to our Open Access Archaeology Fund.