
Cite this as: Bayley, J. 2015, Evidence for Enamelling from Elms Farm, Heybridge, Essex, in M. Atkinson and S.J. Preston Heybridge: A Late Iron Age and Roman Settlement, Excavations at Elms Farm 1993-5, Internet Archaeology 40. http://dx.doi.org/10.11141/ia.40.1.bayley
A strip of opaque red glass was found in a pit dated to the mid-late 1st century AD. Analysis showed it had a composition typical of bright 'sealing-wax' red Roman enamels. It is evidence that enamelling was probably practiced at Heybridge in the 1st century. The types of objects decorated in this way are not known; none of the four hairpins with inlaid heads (Cool type 21) from the site contained glass of this type.

The Roman settlement at Elms Farm, Heybridge was excavated in the early 1990s. Context 3676 (Group 889), in an Early Roman pit located on the northern outskirts of the settlement, was provisionally dated to mid-late 1st century AD. It contained a variety of finds including a strip of opaque red glass (SF173). This was 21mm long and had a rectangular cross-section 12x3mm; it had been cut or broken at both ends from a longer piece of glass. The surface had slight striations along its length, suggesting it had been drawn down from a larger-sectioned block of molten glass (see Figure 716). This report was prepared in 2002.
A small piece of glass was removed from one corner of the strip, set in resin, ground and polished to obtain a flat surface, and examined in a scanning electron microscope (SEM). An energy-dispersive X-ray spectrometer attached to the SEM was used to analyse the composition of the glass.
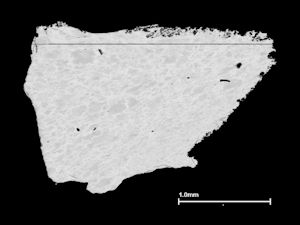
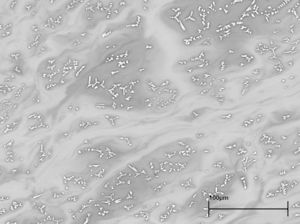
To the naked eye the glass looks homogeneous but the backscattered electron image (Figure 717) shows that there are small-scale variations in composition, with lighter shades of grey indicating areas where the average atomic number is heavier. The structure of the glass can be seen more clearly at higher magnification (Figure 718). Knowing the composition of the glass (Table 199) helps in the interpretation of its features. The small bright areas are crystals of cuprite (copper oxide) that have grown in a dendritic form in the glass melt as it cooled. These crystals give the glass its red colour, and also its opacity. Surrounding the crystals is the heterogeneous lead-rich glass. The cuprite dendrites are concentrated in areas of the glass that appear darker grey in the image. The glass in these areas has a slightly lower lead and copper content relative to the glass further from the crystals, which appears lighter grey in the image. The formation of the cuprite crystals has resulted in localised depletion of the copper in the surrounding glass, and it is possible that the variable lead content of the glass influenced where the crystals formed.
| Heybridge | 'sealing-wax' red | dull red enamel | |
|---|---|---|---|
| (n=3*) | (n=19) | (n=27) | |
| Na20 | 9.1 +0.4 | 10.6 ±2.1 | 12.6 ± 1.7 |
| MgO | 0.6 ± 0.1 | 0.6 ± 0.2 | 2.6 ± 0.5 |
| A1203 | 1.8 ±0.1 | 1.8 +0.3 | 2.3 ±0.4 |
| Si02 | 45.7 ± 0.3 | 41.3 ± 3.6 | 57.4 ± 2.5 |
| 1(20 | 0.3 ± 0.0 | 0.5 ± 0.1 | 1.9 ± 0.5 |
| CaO | 3.6 ± 0.1 | 3.9 ± 0.9 | 7.9 ± 0.9 |
| Ti02 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| FeO | 0.6 ± 0.0 | 0.6 ± 0.3 | 1.9 ± 0.6 |
| CuO | 10.4 ± 0.5 | 6.4 ± 2.0 | 1.8 ± 1.0 |
| PbO | 25.8 ± 0.5 | 32.0 ± 4.2 | 8.2 ± 3.2 |
| Sn02 | nd | 0.4 ± 0.1 | 0.5 ± 0.3 |
| Sb203 | 1.0 ± 0.3 | 1.1 ± 0.5 | 0.3 ± 0.1 |
| MnO | 0.6 ± 0.1 | 0.3 ± 0.2 | 0.5 + 0.2 |
| Total | 100.1 ± 1.1 |
Three areas of the sample seen in Figure 717 were analysed (at 25keV and 1.5µA) and the mean values (± standard deviation) calculated as oxides are given in the first column in Table 199. The results were not normalised as the totals were close to 100%. Note that much of the copper was present as cuprite (Cu20) rather than as cupric oxide (CuO), which the analytical software assumes. For comparison, Table 199 also gives the mean compositions of nineteen analyses of bright 'sealing-wax' red enamels and twenty-seven analyses of dull red enamels from Roman objects carried out by Julian Henderson (1991). The values for the Heybridge strip compare well with the 'sealing-wax' red enamels, showing it to be typical of this type of material.
There is some evidence for non-ferrous metalworking at the site, though little indication of the types of objects being made (Mortimer 1996). Hilary Major has suggested that possible local products are the Cool (1990) Group 21 hairpins with hollow spherical heads containing glass/enamel inserts (pers. comm.). These are a type previously known only from Colchester, but the Heybridge excavations produced four examples, two of which are well stratified and come from contexts consistent with the 1st-2nd century date suggested for the type (Cool 1990).
| Context | Feature | SF No. | Description |
|---|---|---|---|
| 3999 | Spoil heap | 8134 | No inlay survives |
| 4000 | Machining | 970 | No inlay survives |
| 11133 | Pit 11221, Group 226 | 5803 | Some vesicular glass partly fills the depression on the top of the head |
| 13639 | Pit 13640, Group 594 | 6094 | The depression on the top of the head is filled with a lead-rich material, possibly a decayed lead-tin solder |
All of the pins were deeply corroded, so although the results of the XRF analyses were quantitative they could only be used to indicate the broad material types rather than give specific compositions. Analyses of the metal of the pins suggested that they were all basically bronzes (copper + tin), though with variable but minor amounts of zinc and lead. This suggests that the craftsmen making them were aiming for a consistent composition, but that the pins were not made from a single batch of metal. The traces of possible glass set into the heads of two of the pins that had been noted by the finds specialist were also examined and analysed.
The inlay in SF5803 no longer completely filled the depression in the pinhead. It was greenish in colour and had a vesicular (bubbly) structure, suggesting it was indeed glass. If this was the remains of the original inlay, the pin has possibly been strongly heated since the object was made, as a glass inlay (enamel) would not have been this vesicular. XRF analysis of the glass suggested it was deeply corroded and impregnated with copper corrosion products. It is therefore difficult to be certain what its original colour was as all of the colourant elements detected analytically were also detected on the corroded metal surface. However, it is unlikely that the glass in this pinhead was of the same composition as the red enamel strip, as lead was only detected in minor amounts in the glass inlay but was a major component of the strip (see Table 199).
The inlay in SF6094 had expanded as it corroded, parts now standing proud of the top of the pin. Most of it appeared a greenish-grey colour, but there was one area that appeared white. The greenish colour was probably due to corrosion products from the copper-alloy pin. Both white and greenish areas of the inlay were analysed and had very similar compositions. The major element present was lead. The inlay is most unlikely to be a glass. Its expansion as it decayed is typical of metals, so it is most likely that the inlay was lead or a lead-tin solder (traces of tin were detected by XRF). Whether the intention of the craftsman who made this pin was just to provide a colour contrast - the white/silver appearance of lead or solder against the brown of the bronze - or whether the inlay originally served to attach some other material to the pinhead cannot now be discovered, although parallels for both can be found in early Roman copper-alloy objects. Colour contrasts are common on 1st-century brooches such as the Hod Hill series, while attached silver foils are well known on early military studs etc.
The strip of red glass was almost certainly intended to be used as enamel, to decorate copper-alloy objects. It would have been one of the raw materials used by an enameller or metalworker and thus indicates that enamelling was one of the crafts likely to have been carried out at Heybridge. The form of the find is unparalleled; raw glass that could have been used for enamelling is usually found as cuboid tesserae or amorphous lumps. Both are known from Castleford (Cool and Price 1998, 193) and 'crude lumps of green enamel' were described by Wheeler (1946, 32) from Nicholas Lane, London.
The types of objects being enamelled are not known. The original suggestion that the Group 21 pins were both made and enamelled on the site is still possible, though there is no specific evidence to support this hypothesis. Certainly red glass of the composition of the enamel strip did not survive in any of the pins. In the two where some inlay was present, one was glass/enamel of indeterminate colour but the other inlay appeared to have been a lead-rich metal.
Internet Archaeology is an open access journal based in the Department of Archaeology, University of York. Except where otherwise noted, content from this work may be used under the terms of the Creative Commons Attribution 3.0 (CC BY) Unported licence, which permits unrestricted use, distribution, and reproduction in any medium, provided that attribution to the author(s), the title of the work, the Internet Archaeology journal and the relevant URL/DOI are given.
Terms and Conditions | Legal Statements | Privacy Policy | Cookies Policy | Citing Internet Archaeology
Internet Archaeology content is preserved for the long term with the Archaeology Data Service. Help sustain and support open access publication by donating to our Open Access Archaeology Fund.